Novel 3-(4-amino-1,3-dihydro-1-oxo-2H-isoindole-2-yl)-2,6-piperidinedione crystal forms and preparation method thereof
A technology of piperidinedione and piperidinedione salt is applied in the field of preparation of new crystal forms, and can solve the problems of long cycle, large amount of solvent, high energy consumption and cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
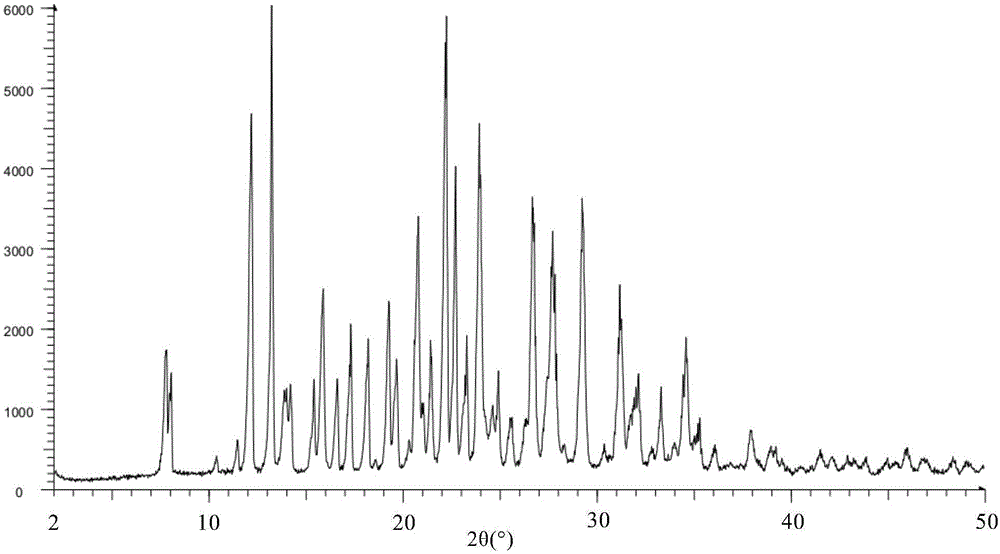
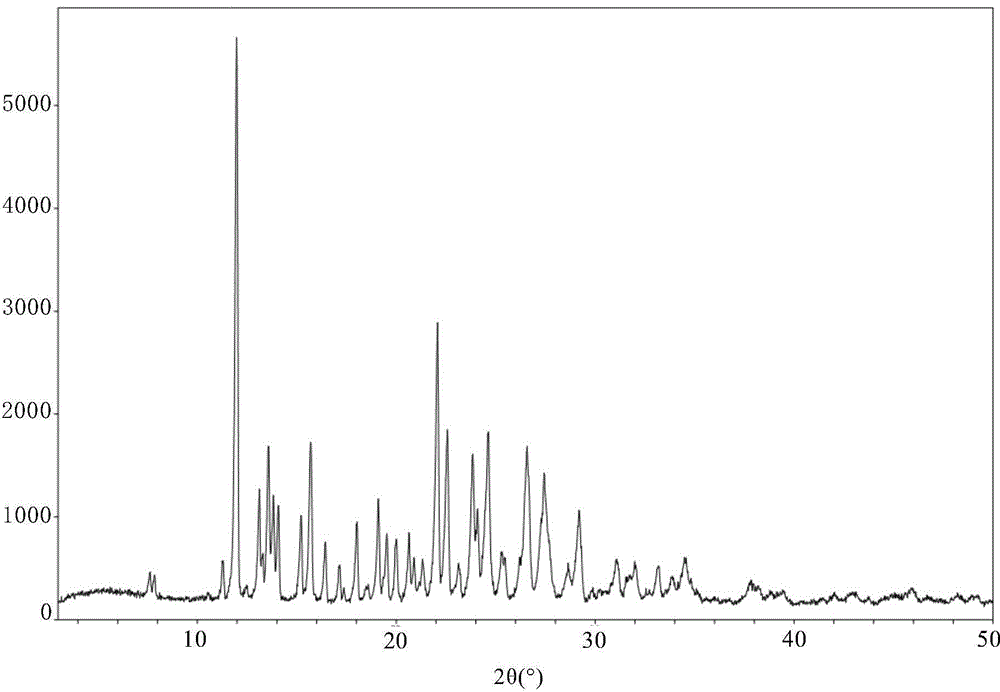
[0194] Preparation of new crystal form X of 3-(4-amino-1,3-dihydro-1-oxo-2H-isoindol-2-yl)-2,6-piperidinedione
[0195] Mix 43g of 3-(4-amino-1,3-dihydro-1-oxo-2H-isoindol-2-yl)-2,6-piperidinedione methanesulfonate raw material with 690ml of deionized water, Add it into a 1L three-necked flask, stir and mix well at room temperature until the solute is completely dissolved; filter the solution under reduced pressure, and transfer the resulting clear solution into another 2L three-necked flask. Adjust the mechanical stirrer, add dropwise 400ml of aqueous sodium bicarbonate solution with a concentration of 0.525mol / L, and start the reaction crystallization process; after dropping, under the protection of nitrogen, raise the temperature of the system to 80-82°C and keep it warm for 30 hours, then lower the temperature of the system to 53~56°C, filter the crystalline slurry with suction, wash the filter cake three times with a certain volume of deionized water at about 55°C; vacuum...
Embodiment 2
[0199] Preparation of new crystal form X of 3-(4-amino-1,3-dihydro-1-oxo-2H-isoindol-2-yl)-2,6-piperidinedione
[0200] Mix 30g of 3-(4-amino-1,3-dihydro-1-oxo-2H-isoindol-2-yl)-2,6-piperidinedione methanesulfonate raw material with 750ml of deionized water, Add it into a 1L three-necked flask, stir and mix well at room temperature until the solute is completely dissolved; filter the solution under reduced pressure, and transfer the resulting clear solution into another 2L three-necked flask. Adjust the mechanical stirrer, add dropwise 300ml of aqueous sodium bicarbonate solution with a concentration of 0.525mol / L, and start the reaction crystallization process; after dropping, under the protection of nitrogen, raise the temperature of the system to 80-83°C and keep it warm for 28 hours, then lower the temperature of the system to 55~58°C, filter the crystalline slurry with suction, wash the filter cake twice with a certain volume of deionized water at about 55°C; dry the filt...
Embodiment 3
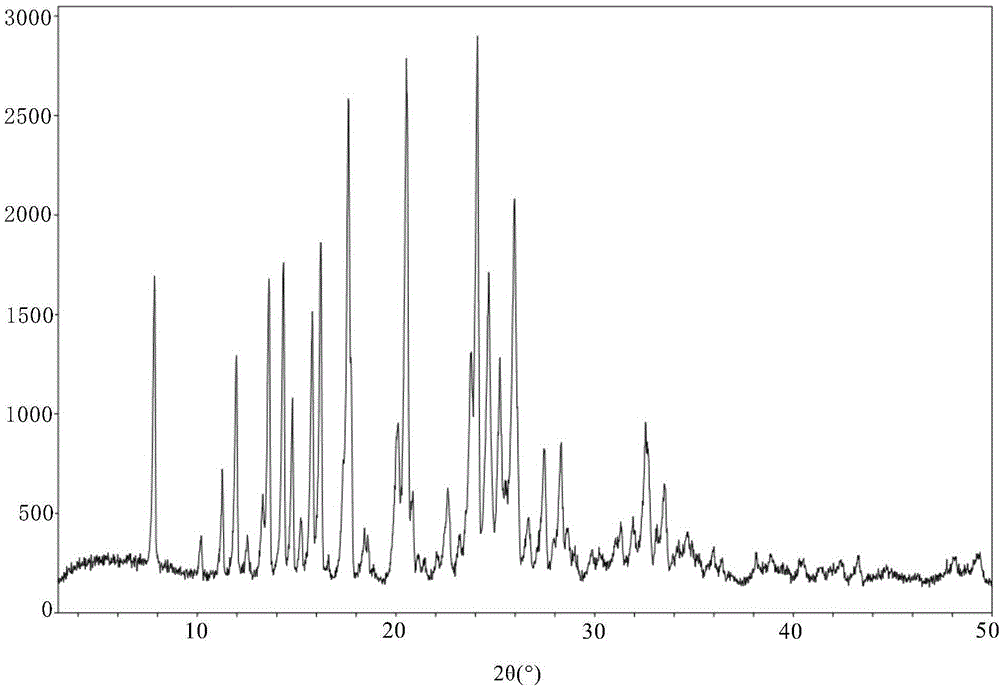
[0203] Preparation of new crystal form XI of 3-(4-amino-1,3-dihydro-1-oxo-2H-isoindol-2-yl)-2,6-piperidinedione
[0204] The volume ratio of 37g 3-(4-amino-1,3-dihydro-1-oxo-2H-isoindol-2-yl)-2,6-piperidinedione methanesulfonate raw material to 400ml is 20 : 80 deionized water / methanol mixed solvent, add in a 1L three-necked flask, stir and mix well at room temperature until the solute is completely dissolved; filter the solution under reduced pressure, and transfer the resulting clear solution into another 1L three-necked flask. Stir fully, slowly add triethylamine to the solution, and start the reaction crystallization process; during the dropwise addition, a large amount of off-white solids precipitate; when the pH of the system is adjusted to 8.3, under nitrogen protection, stir at room temperature for 4 hours, and filter the crystal slurry , the filter cake was washed 3 times with deionized water; the filter cake was vacuum-dried for 20 hours at room temperature to obtain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bulk density | aaaaa | aaaaa |
| Bulk density | aaaaa | aaaaa |
| Bulk density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com