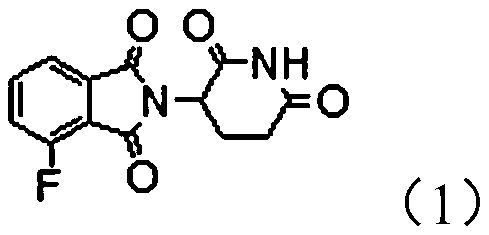
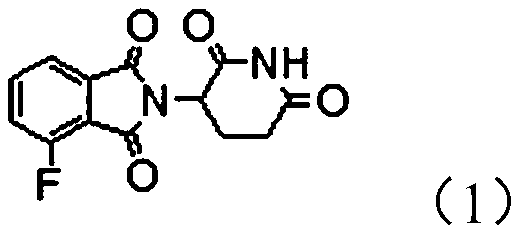
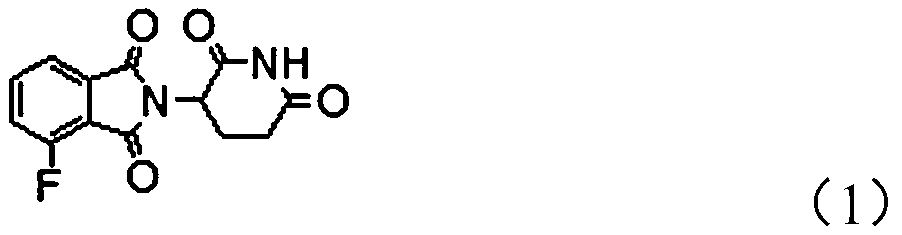Protein degradation targeting chimeric compound, application and preparation method thereof
A protein degradation and chimera technology, applied in and its preparation, protein degradation targeting chimera compounds, application fields, can solve the problems of difficulty in entering cells, large molecules of polypeptide PROTACs, etc., achieve small molecular weight, good leaving performance, good size effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] As the method for preparing the above-mentioned protein degradation targeting chimera compound, it may include the following steps:
[0034] Step S1: fluorination reaction of 3-chlorophthalic anhydride to generate 3-fluorophthalic anhydride.
[0035] Specifically, the reaction equation can be as shown in the following equation (2):
[0036]
[0037] More specifically, it can generate 3-fluorophthalic anhydride by performing a chlorination reaction and then a fluorination reaction. In other words, the fluorination process may include the following steps:
[0038] Step S11, dissolving 3-chlorophthalic anhydride in sulfolane, and adding a chlorinating reagent to cause a chlorination reaction.
[0039] Further, the chlorinating reagent is oxalyl chloride, and the chlorination reaction is performed at 80-120°C, and the reaction time may be, for example, 2-6 hours.
[0040] Step S12, after the chlorination reaction is completed, the fluorination reaction occurs with potassium fluoride ...
Embodiment 1
[0059] In a 1L three-necked flask, add 500ml of sulfolane, then add 180g of 3-chlorophthalic anhydride to it, cool down to below 10°C in an ice bath, add 300g of oxalyl chloride dropwise to it, and after the dropwise addition, heat to about 100°C and react for 3 hours, then cool to At room temperature, remove oxalyl chloride by rotary evaporation. Then 180g of anhydrous potassium fluoride was added to it, after adding potassium fluoride, the system was heated to 100°C for 6 hours, and then cooled to room temperature. The inorganic salt was filtered off with suction. The filtrate was slowly poured into 900ml of ice water to precipitate a large amount of solids. Stirring is continued for 2 hours, and filtered with suction to obtain a filter cake, which is vacuum dried to obtain crude 3-fluorophthalic anhydride.
[0060] The crude 3-fluorophthalic anhydride was dissolved in 800ml of dichloromethane, dried with anhydrous magnesium sulfate, filtered with suction, the filter cake was s...
Embodiment 2
[0066] In a 1L three-necked flask, add 500ml of sulfolane, then add 180g of 3-chlorophthalic anhydride to it, cool down to below 10°C in an ice bath, add 360g of phosphorus oxychloride dropwise to it, after the dropwise addition, heat up to about 100°C for 3 hours. Cool to room temperature and remove phosphorus oxychloride by rotary evaporation. Then 180g of anhydrous potassium fluoride was added to it, after adding potassium fluoride, the system was heated to 100°C for 6 hours, and then cooled to room temperature. The inorganic salt was filtered off with suction. The filtrate was slowly poured into 900ml of ice water to precipitate a large amount of solids. Stirring is continued for 2 hours, and filtered with suction to obtain a filter cake, which is vacuum dried to obtain crude 3-fluorophthalic anhydride.
[0067] The crude 3-fluorophthalic anhydride was dissolved in 800ml of dichloromethane, dried with anhydrous magnesium sulfate, filtered with suction, the filter cake was spu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com