Improved process
一种来那度胺、硝基的技术,应用在制备3--哌啶-2领域,能够解决低产率、差可行性、危险等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
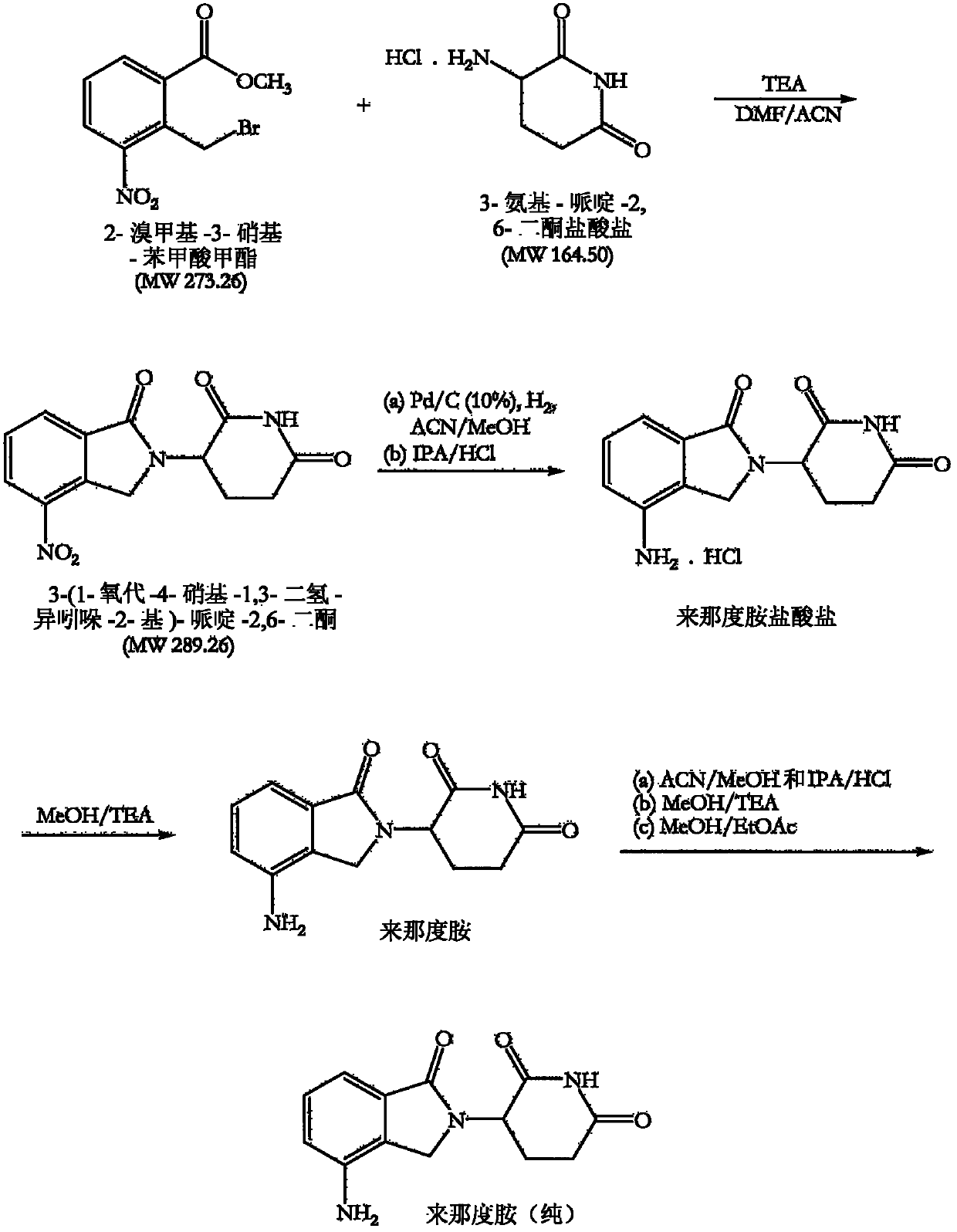
[0140] Example 1 : Preparation of 3-(1-oxo-4-nitro-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione
[0141]Add triethylamine (140.8g, 2.29mol) to 3-amino-piperidine-2,6-dione hydrochloride (100g, 0.61mol) in N,N-dimethylformamide at 25-30°C (800ml) in solution. A solution of methyl 2-bromomethyl-3-nitro-benzoate (186.0 g, 1.13 mol) in acetonitrile (200 ml) was then added under stirring and nitrogen atmosphere and the reaction mixture was heated to 50-55°C, Lasts for 8-10 hours. After completion of the reaction, approximately 60% of the solvent was removed by distillation under reduced pressure (100 mbar) at 50-60°C. Water (1000ml) was added to the remaining mixture at 50-55°C and stirred at this temperature for 1 hour. The obtained solid product was cooled to 25-30°C and filtered. The solid was washed with water (500ml) at 50-55°C, cooled at ambient temperature and filtered again. Finally, the solid was washed with methanol (500 ml) at 50-55°C, cooled at 25-30°C and fil...
Embodiment 2
[0144] Example 2 : Preparation of 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione (I) (lenalidomide)
[0145] Dissolve 3-(1-oxo-4-nitro-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione (100 g, 0.35 mol) in acetonitrile and methanol mixture (7000ml, 1:1, v / v). A catalyst comprising a slurry of 10% palladium on carbon (5.0 g) in methanol (25 ml) with a water content of approximately 50% was added under a nitrogen atmosphere. The nitrogen was replaced with hydrogen and bubbled through the reaction mixture with stirring. The reaction mixture was maintained at 30-35°C. After 1 hour, a second amount of catalyst was added and hydrogen sparging was continued until the reaction was complete after a total time of about 2-2.5 hours. The completion of the reaction was monitored by TLC. The catalyst was completely removed by filtration. The filtrate was concentrated and approximately two-thirds of the solvent was removed by distillation at a temperature of 45-50°C an...
Embodiment 3
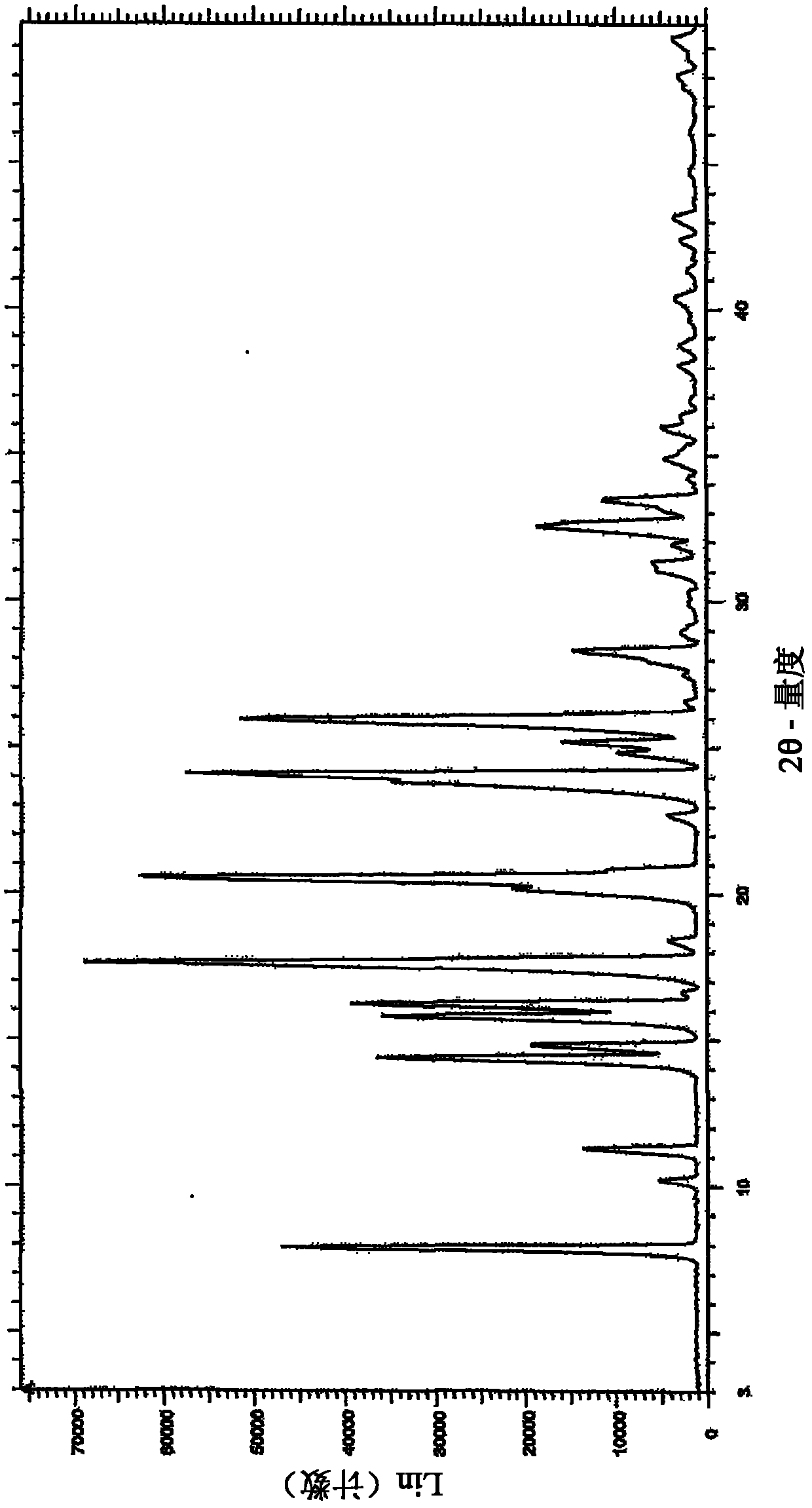
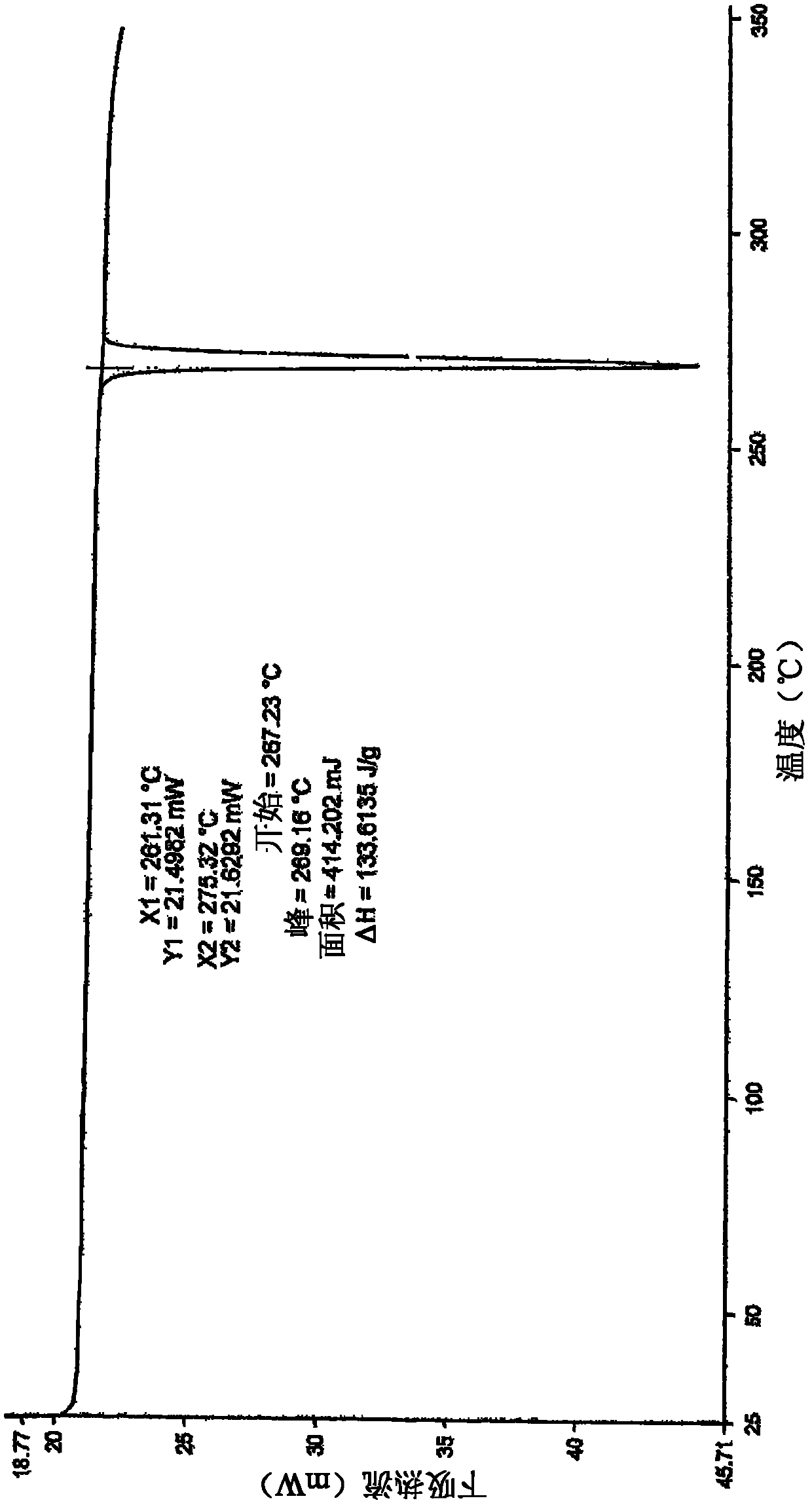
[0149] Example 3 : Further purification of lenalidomide
[0150] Lenalidomide was prepared as described above.
[0151] Lenalidomide (70 g) was suspended in a mixture of acetonitrile and methanol (700 ml, 1:1, v / v) and an equimolar portion of a crude lenalidomide in hydrogen chloride solution in isopropanol was added slowly. The reaction mixture was stirred at ambient temperature for 45 minutes, then slowly cooled to 5-10 °C and held at this temperature for 45 minutes. The precipitated solid was filtered and dried. The solid was washed in methanol (100ml) at ambient temperature and filtered, then dried to remove all solvent.
[0152] Lenalidomide hydrochloride was suspended in methanol (350 ml), and 1.1 molar equivalents of triethylamine were slowly added with stirring. The reaction mixture was stirred at ambient temperature for 45 minutes, then cooled at 5-10 °C and maintained at this temperature for a further 45 minutes. The precipitated solid was filtered and dried. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com