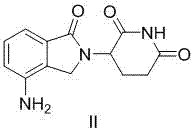
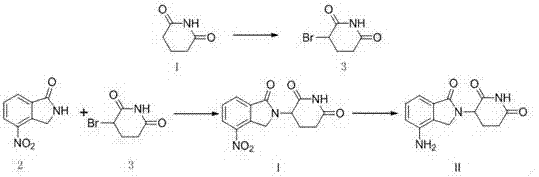
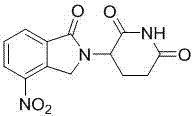
Preparation method of lenalidomide
A technology of lenalidomide and compounds, which is applied in the field of drug synthesis, can solve the problems of iron sludge difficult to filter and iron ion contaminated products, and achieve the effects of reducing production costs and cycles, facilitating industrialized production, and realizing industrialized production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of 3-(4-nitro-1-oxo-1,3-dihydro-2H-isoindol-2-yl)piperidine-2,6-dione (I):
[0031]
[0032] Add 99.9g of 2-bromomethyl-3-nitrobenzoic acid methyl ester, 50.0g of 3-amino-2,6-piperidinedione hydrochloride and 96.6g of sodium carbonate to 500ml of tetrahydrofuran, heat to reflux for reaction, HPLC Stop the reaction when it detects that 3-amino-2,6-piperidinedione hydrochloride is less than or equal to 0.5%, cool the reaction solution to room temperature, pour the reaction solution into 2L purified water, stir at room temperature for 1 hour, filter, and the filter cake is dispersed in In a mixture of 1L ethanol and 1L purified water, stir at room temperature for 1 hour, filter, disperse the filter cake in 1L ethanol, stir for 1 hour at room temperature, filter, wash the filter cake once with 200ml ethanol, and dry the filter cake at 50°C for 10 Hours, 80.8 g of an off-white solid was obtained, with a yield of 92.0%. 1 HNMR (DMSO) δ11.03(S, 1H), 8.46(dd, 1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com