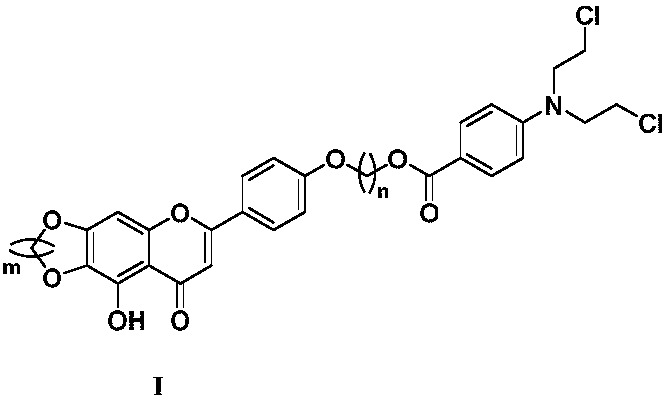
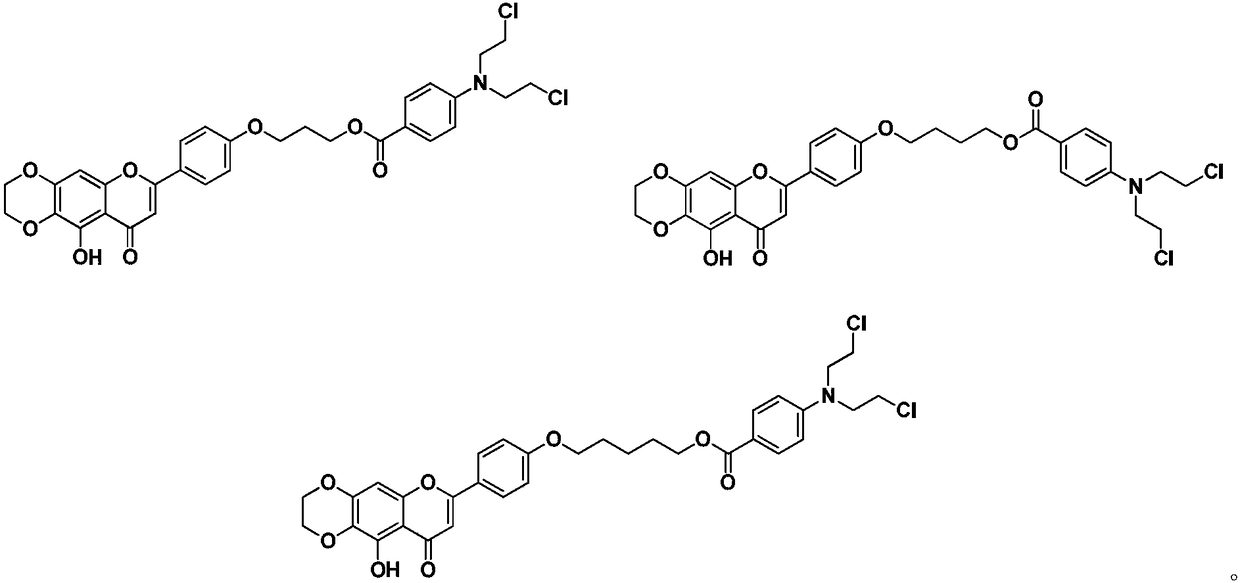
Benzoic acid nitrogen mustard fragment-containing compound and preparation method and use thereof
A technology of chlorambucil and compound, which is applied in the field of medicinal chemistry and can solve problems such as large toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
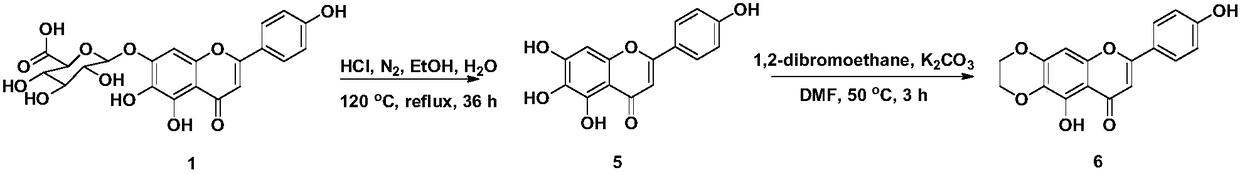
[0026] Add scutellarin 1 (10g, 21.6mmol) to 120mL of absolute ethanol, 120mL of concentrated hydrochloric acid and 10mL of H 2 O in the mixture. In N 2 Under protection conditions, reflux for 36 hours. After cooling at room temperature, the reaction solution was poured into an equal volume of water, filtered with suction, washed with water until neutral, dried, and the crude product was separated by silica gel column chromatography (petroleum ether: ethyl acetate 1:1) to obtain yellow solid scutellarin glycoside Yuan 5 1.05g, the yield is 17%. 1 H NMR(DMSO-d 6 ,400MHz)δ(ppm): 12.80(s,1H,5-OH), 10.47(s,1H,7-OH), 10.32(s,1H,4′-OH), 8.75(s,1H,6- OH),7.91(d,2H,J=8.9Hz,H-2′,6′),6.92(d,2H,J=8.9Hz,H-3′,5′),6.75(s,1H,H -8), 6.57(s, 1H, H-3).
Embodiment 2
[0028]
[0029] Dissolve 5 (286mg, 1mmol) in 5mL DMF, add K 2 CO 3 (207 mg, 1.5 mmol) and 1,2-dibromoethane (130 μL, 1.5 mmol). In N 2 Protected, reacted for 3h at 115℃. After cooling to room temperature, the reaction solution was poured into 50 mL of H 2 Extract with ethyl acetate (3×30 mL) in O, wash with saturated aqueous salt solution, dry with anhydrous sodium sulfate, filter, concentrate the filtrate, and separate by silica gel column chromatography (petroleum ether: ethyl acetate 3:1) to obtain light yellow powder 6 90mg, yield 29%. 1 H NMR(DMSO-d 6 ,400MHz)δ(ppm): 13.03(s,1H,5-OH),10.37(s,1H,4′-OH),7.93(d,2H,J=8.7Hz,H-2′,6′) ,6.92(d,2H,J=8.7Hz,H-3′,5′),6.80(s,1H,H-8),6.73(s,1H,H-3),4.39(t,2H,J =4.7Hz,-CH 2 -), 4.29(t,J=4.7Hz,2H,-CH 2 -).
Embodiment 3
[0031]
[0032] Intermediate 6 (312 mg, 1 mmol) was dissolved in 30 mL of acetone, and K was added 2 CO 3 (417mg, 3mmol) and 1,3-dibromopropane (420μL, 3mmol) were refluxed for 8h. After cooling to room temperature, it was filtered with suction, the filtrate was concentrated, and separated by silica gel column chromatography (petroleum ether: ethyl acetate 4:1) to obtain 290 mg of light yellow powder 7a with a yield of 67%. Intermediate 7a (108mg, 0.25mmol) was dissolved in 5mL DMF, and K was added 2 CO 3 (68mg, 0.5mmol) and chlorambucil 4 (76mg, 0.25mmol) were reacted at room temperature for 24h. Pour the reaction solution into 30mL of H 2 Extract with ethyl acetate (3×20 mL) in O, wash with saturated aqueous salt solution, dry with anhydrous sodium sulfate, filter, concentrate the filtrate, and separate by silica gel column chromatography (petroleum ether: ethyl acetate 4:1) to obtain light yellow powder 8a55mg, yield 36%. 1 H NMR(DMSO-d 6 , 400MHz)δ(ppm): 12.32(s,1H,5-OH),8....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com