Combination therapy with an anti - CD19 antibody and a nitrogen mustard
An antibody and sequence technology, applied in the field of combined therapy using anti-CD-19 antibody and nitrogen mustard, can solve problems such as poor prognosis of cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0075] One aspect of the present disclosure includes a CD19-specific antibody in combination with a nitrogen mustard for the treatment of non-Hodgkin's lymphoma, chronic lymphocytic leukemia, and / or acute lymphoblastic leukemia. In an embodiment, the combination is synergistic.
[0076] Here, the exemplified combination of an anti-CD19 antibody and bendamustine acts synergistically in in vitro and in vivo models associated with NHL and CLL. Since both NHL and CLL are B cell related disorders and CD19 is highly expressed on B cells, the exemplified combination should have the same mechanism of action in the treatment of other B cell related disorders such as ALL and should also work synergistically effect. Thus, combinations of the exemplified CD19-specific antibodies with bendamustine will be effective in the treatment of human non-Hodgkin's lymphoma, chronic lymphocytic leukemia and / or acute lymphoblastic leukemia.
[0077] Since the mechanism of action of bendamustine and ...
Embodiment 1
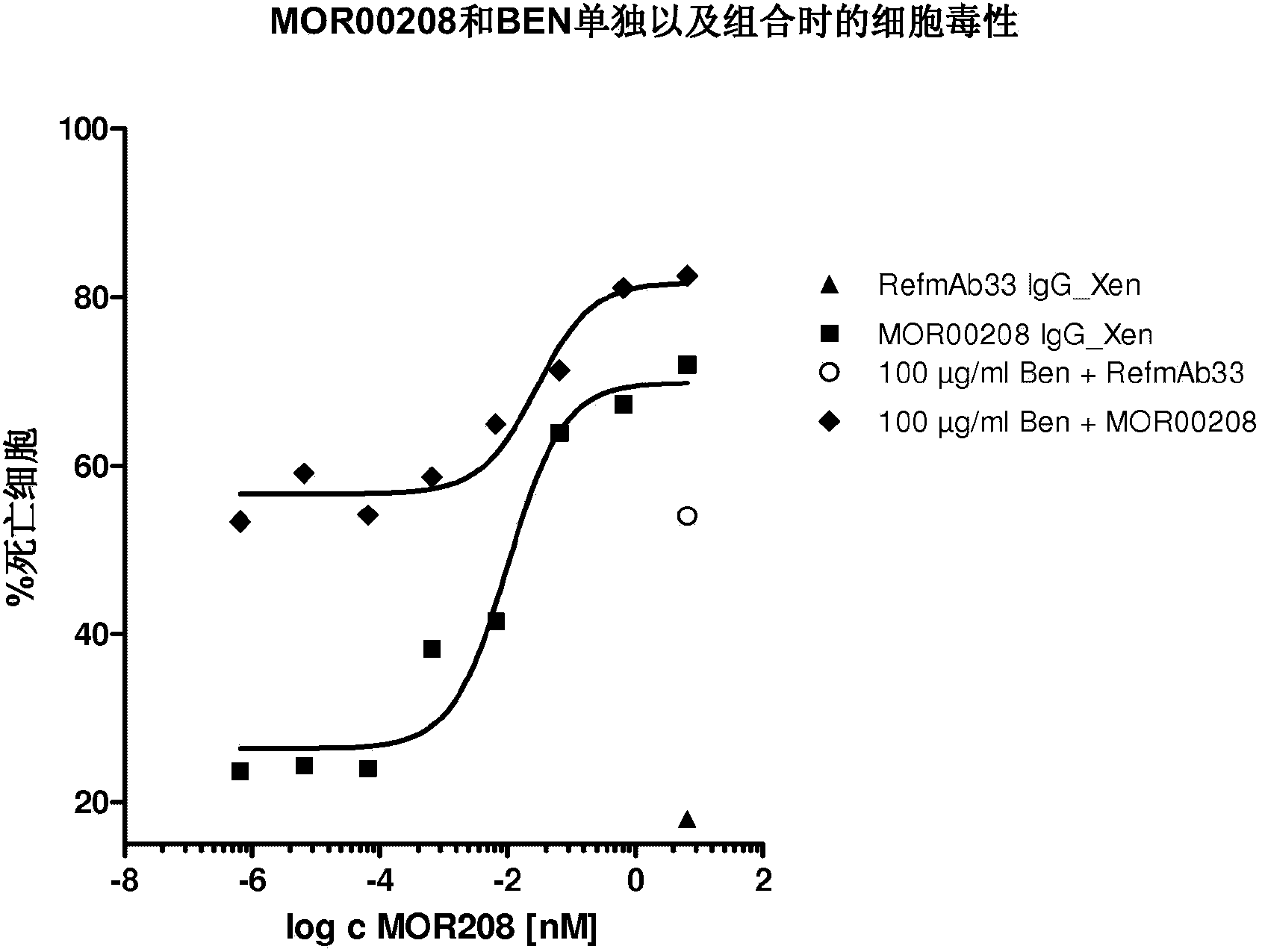
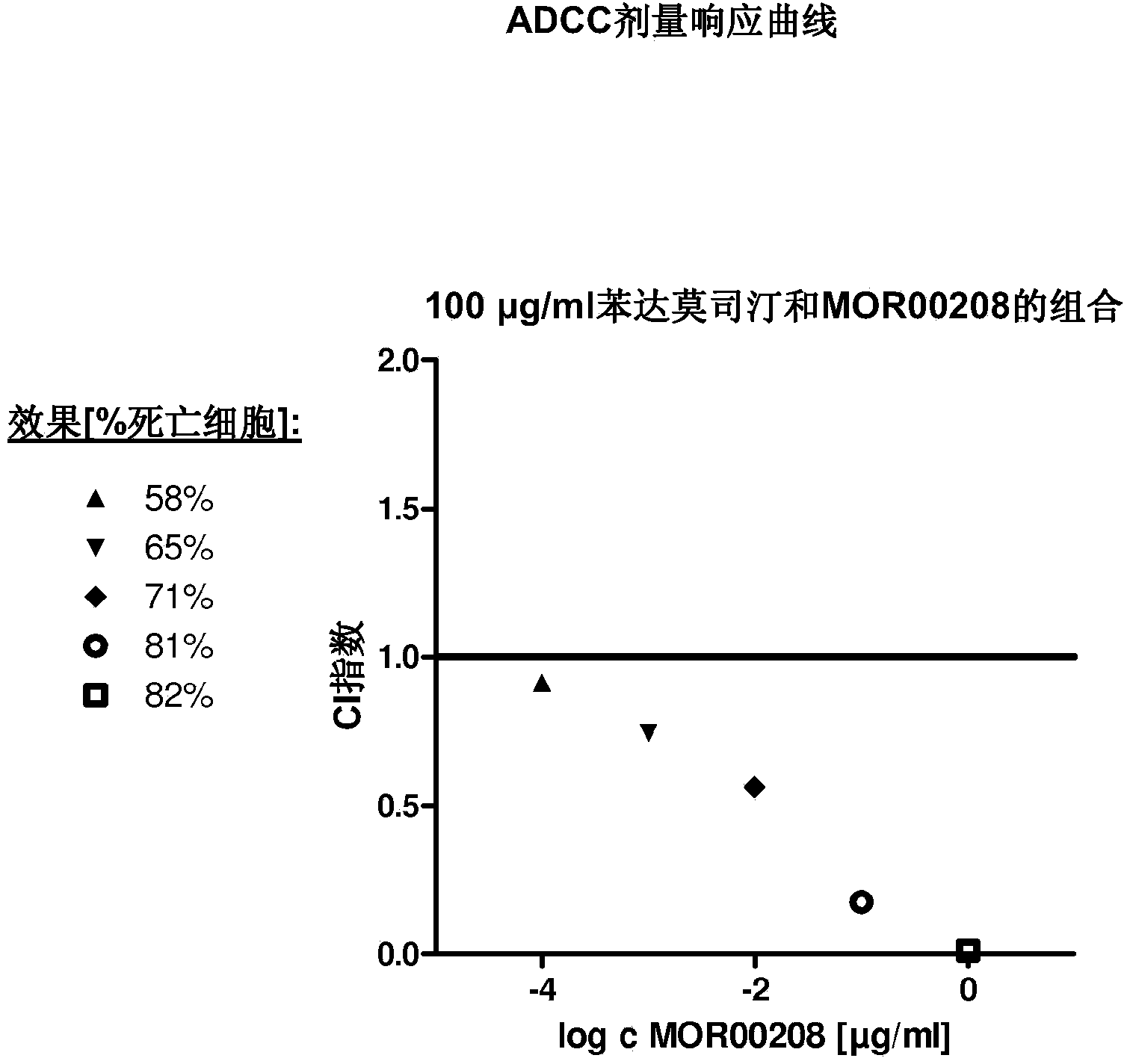
[0092] Example 1 : MOR00208 and bendamustine alone and in combination inhibit the proliferation of MEC-1 cells
[0093] Material
[0094] MEC-1 cells: chronic B-cell leukemia cell line DSMZ#ACC497; cell culture medium: with GlutaMAX TM Iscove's Modified Dulbecco's Medium (IMDM), Invitrogen, Cat No.: 31980-048, 20% FCS; PBMC: RPMI1640 with stabilized glutamine, PAN Biotech GmbH, Cat No.: P04-13500 supplemented with 10 %FCS; Biocoll: Biochrome AG CAT No.:L6115LOT No.:1050T; Bendamustine: Mundipharma LOT No.:88018; FCS: PAN CAT No.:3302-P282403LOT No.:P282403; and RefmAb33 (anti- RSV), the Fc region is the same as MOR00208.
[0095] method
[0096] The cytotoxicity of MOR00208 and bendamustine alone and in combination was tested in MEC-1 cells. BEN is an alkylating agent and thus acts via direct cytotoxicity in MEC-1 cells. MOR00208 targets CD19 and additionally functions via ADCC in the killing of MEC-1 cells. MEC-1 cell killing was measured in the following groups: ...
Embodiment 2
[0125] MOR00208 and BEN alone and in combination were subcutaneously (SC)-implanted into a human Ramos Burkitt's B-cell lymphoma tumor growth model.
[0126] Material
[0127] RAMOS human Burkitt's lymphoma cells (ATCC number CRL-1596, lot #3953138); vehicle control: 150mM NaCl, 25mg / mL mannitol, pH5.5-6.0; (adjusted with 0.01M NaOH). Ref_mAb_33_IgG_Xen (10 mg / mL in PBS, called Ref_mAb_33). Six-week-old female C.B-17SCID mice (CB17 / lcr-Prkdcscid / lcrlcoCrl) were purchased from Charles River Laboratories (Wilmington, MA) and acclimatized in the laboratory for 9 days before experiments.
[0128] method
[0129] SCID mice were subcutaneously implanted with RAMOS cells (~5x10 6 cells / mouse). When the mouse has a size of about 150mm 3 , or ~14 days after inoculation, they were divided into groups, where each group had tumor volumes that were more similar in size. Treatment started on day 15. Treatment regimens are provided in Table 4. The study duration was 60 days.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com