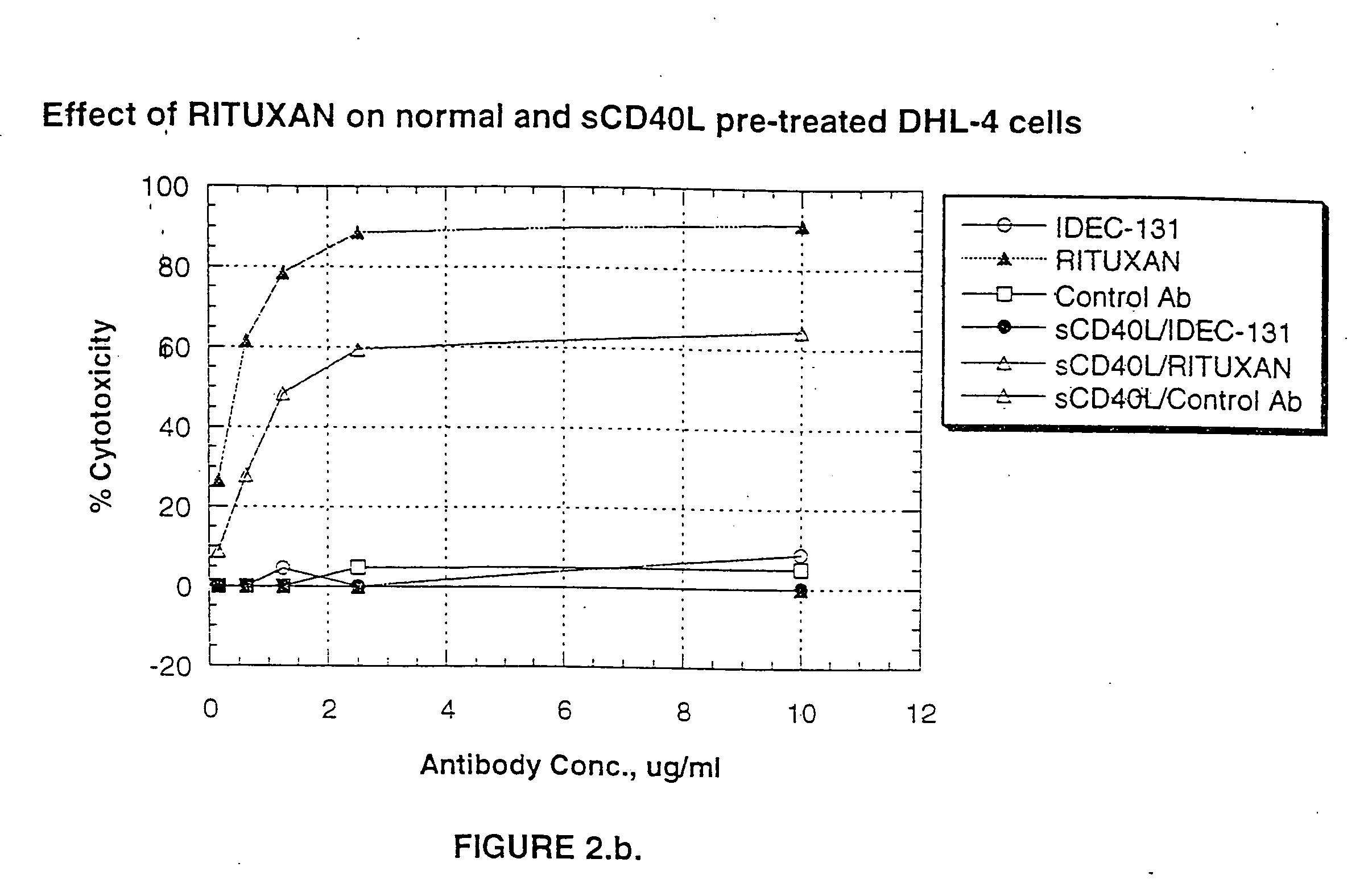
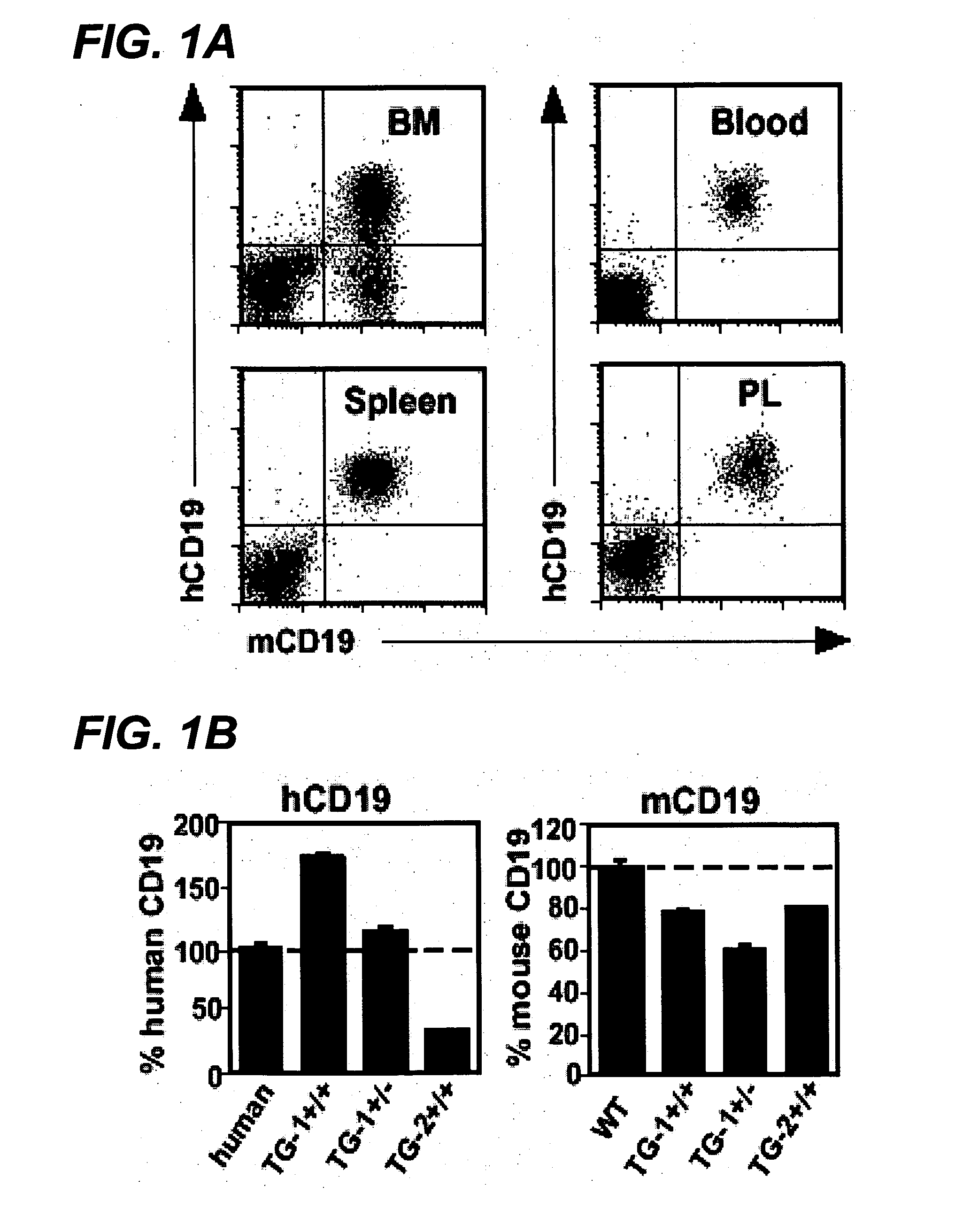
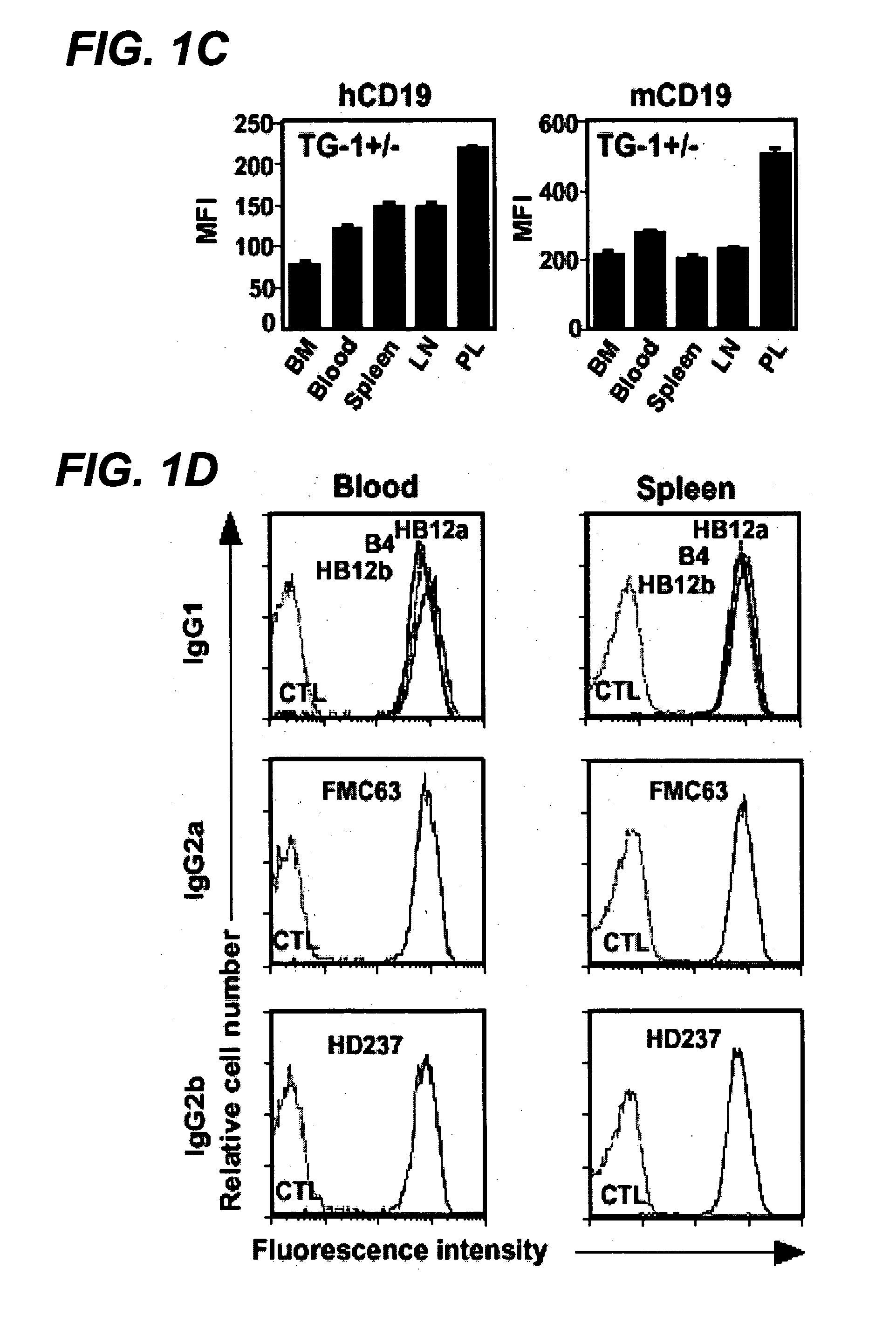
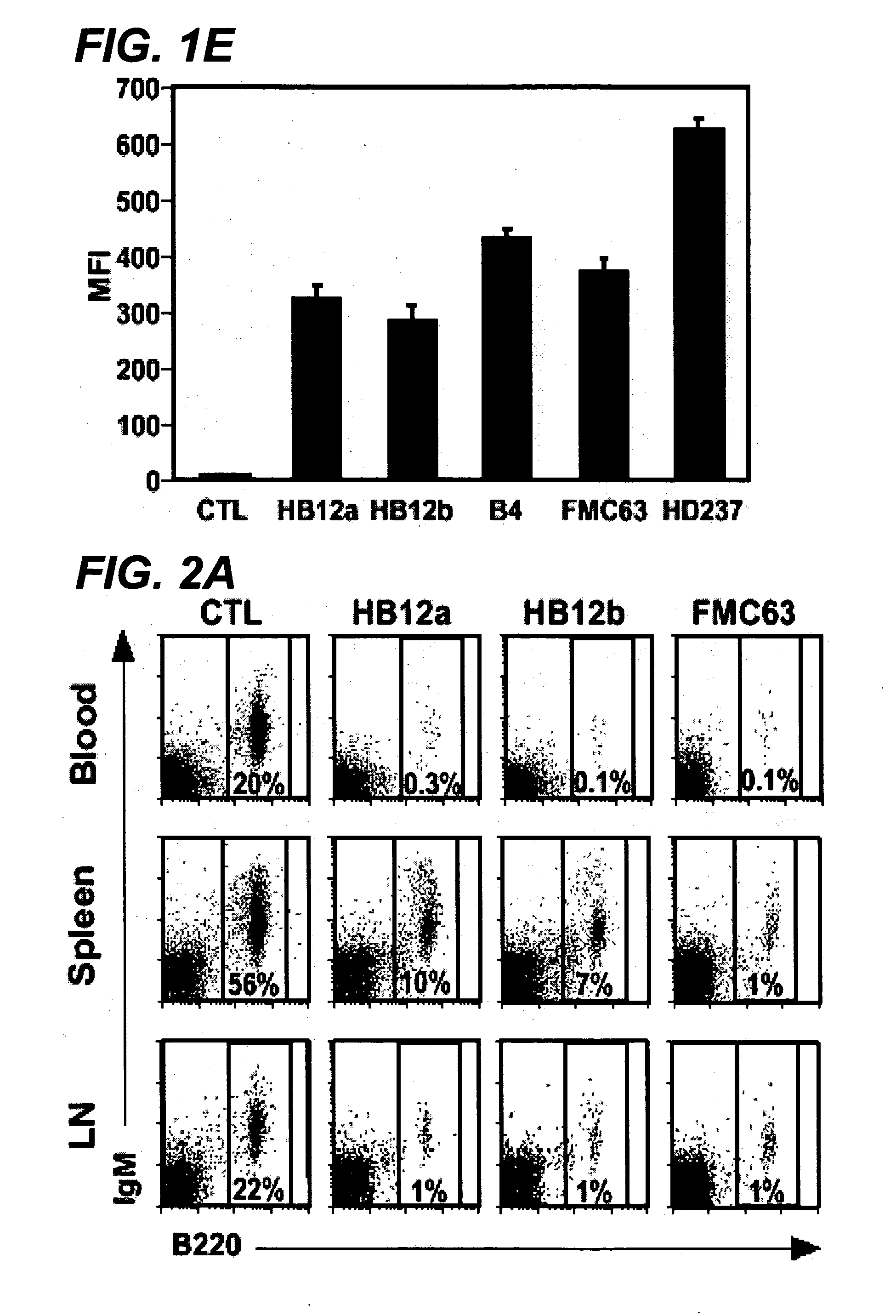
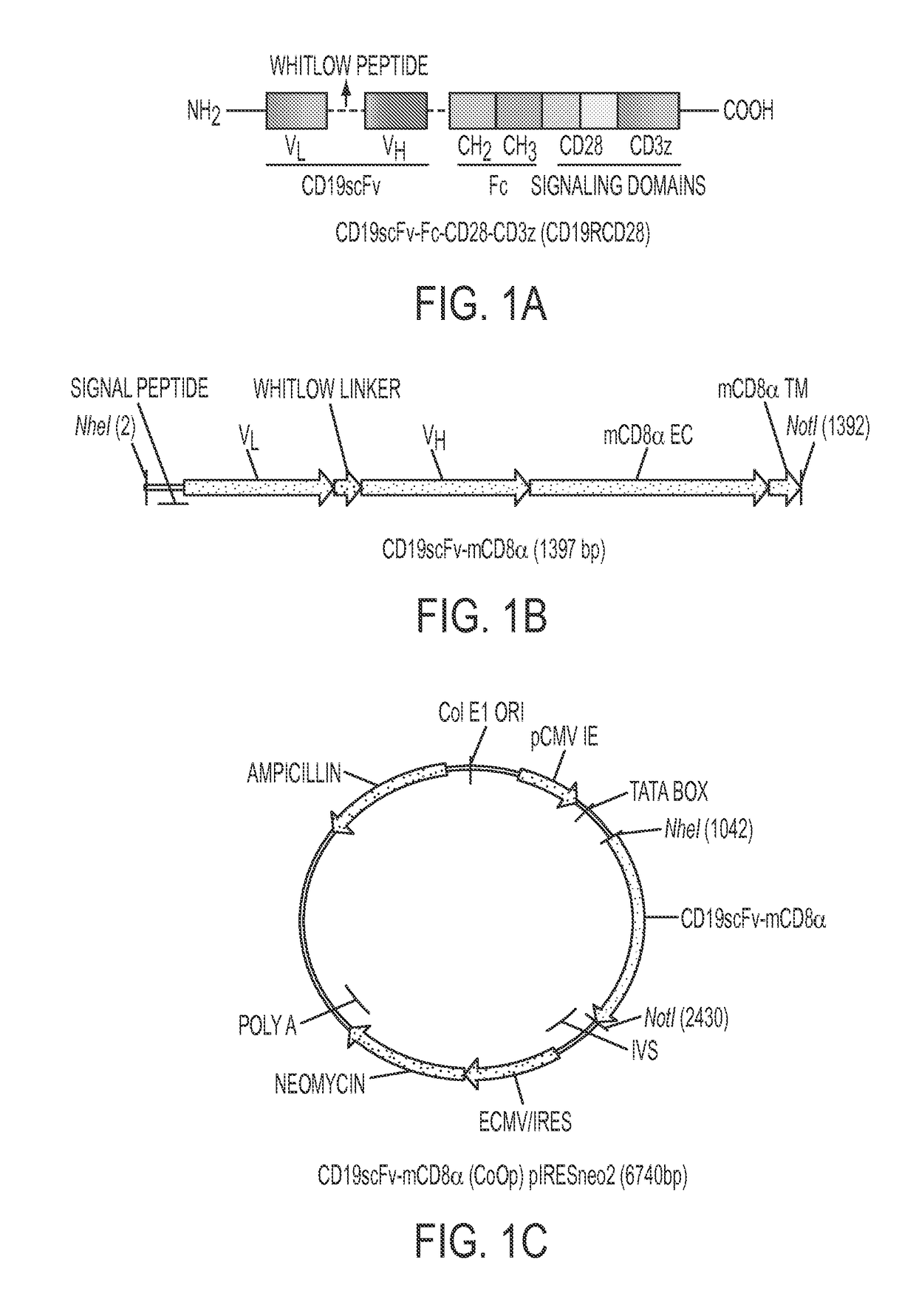
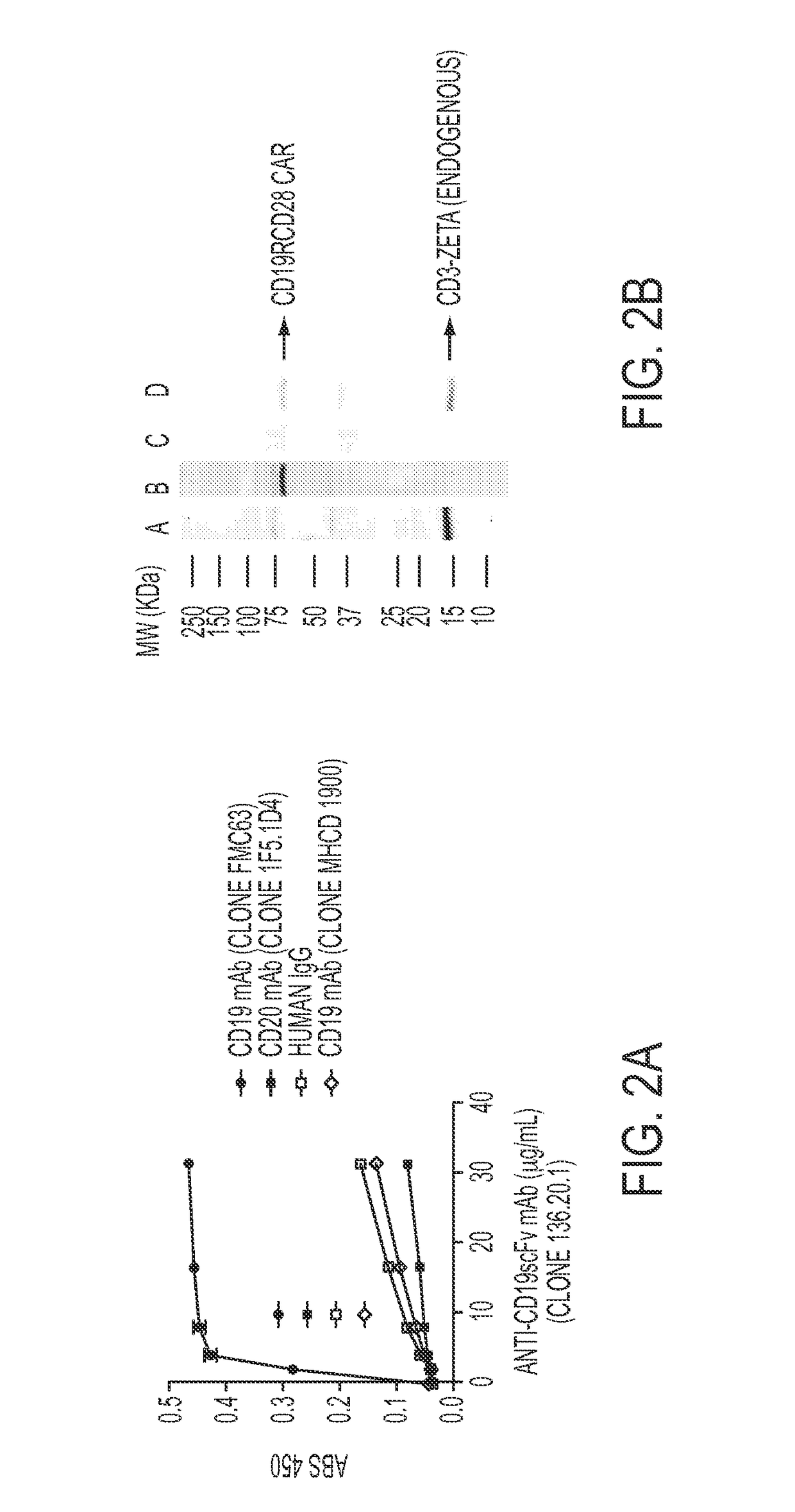
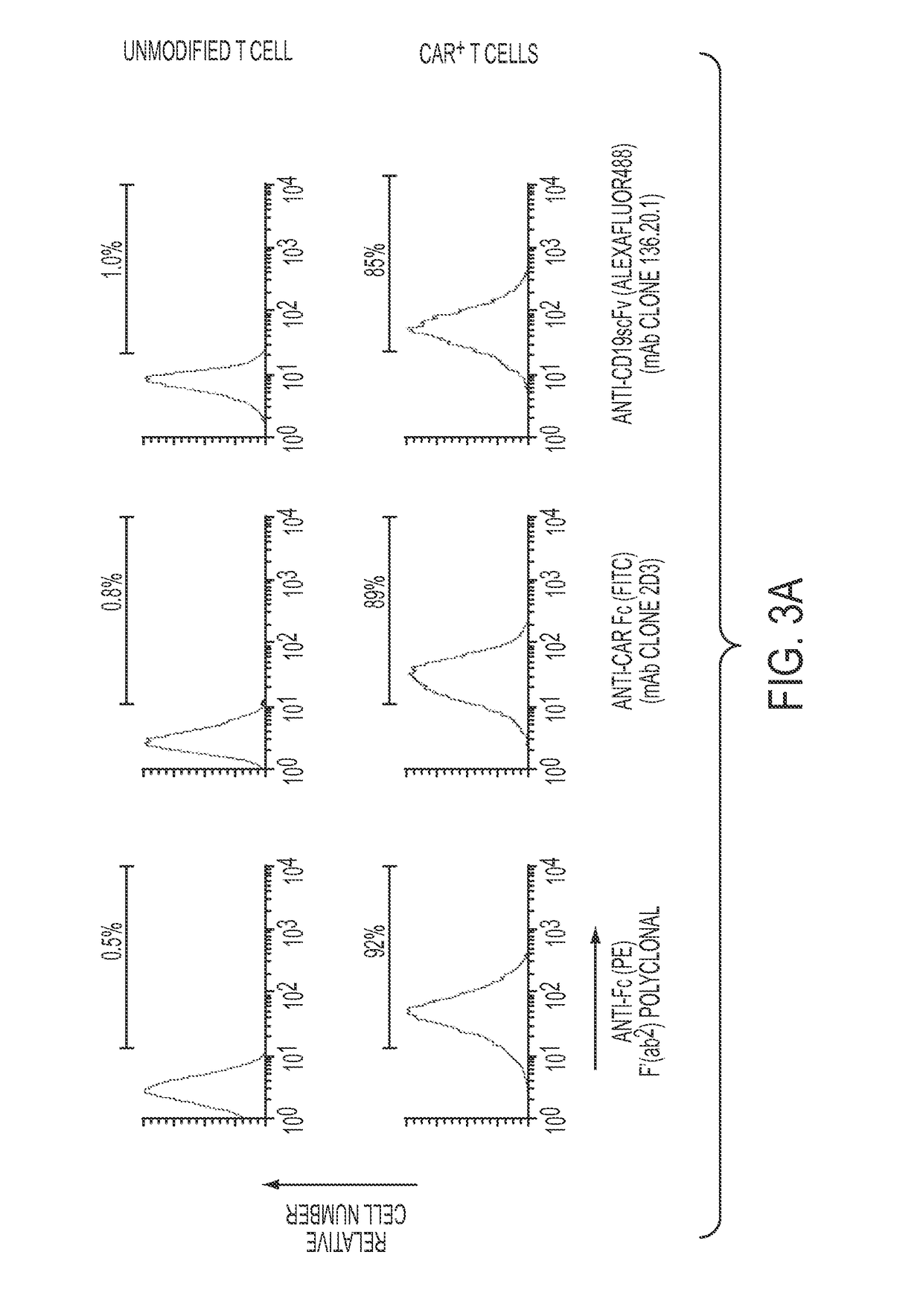
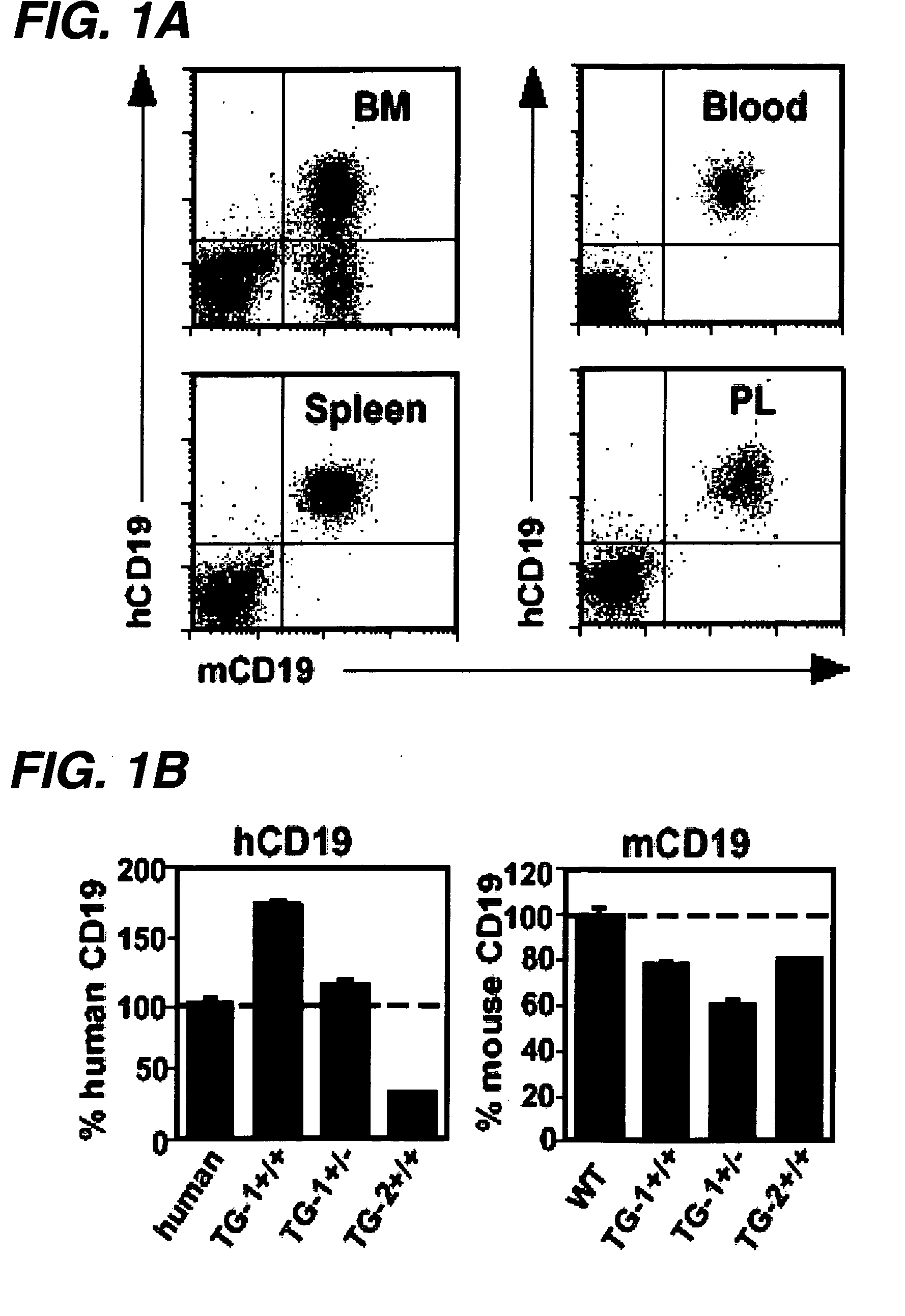
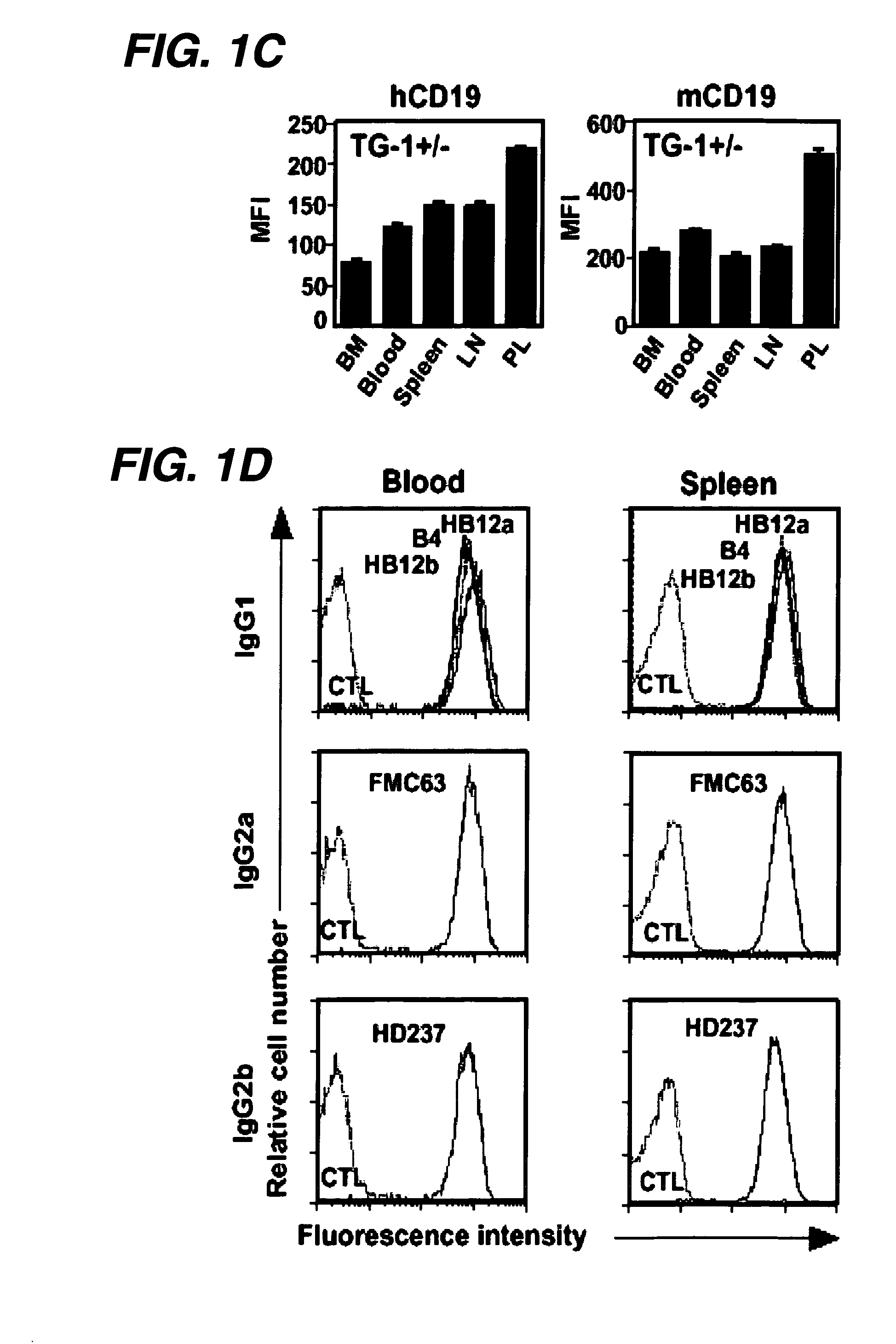
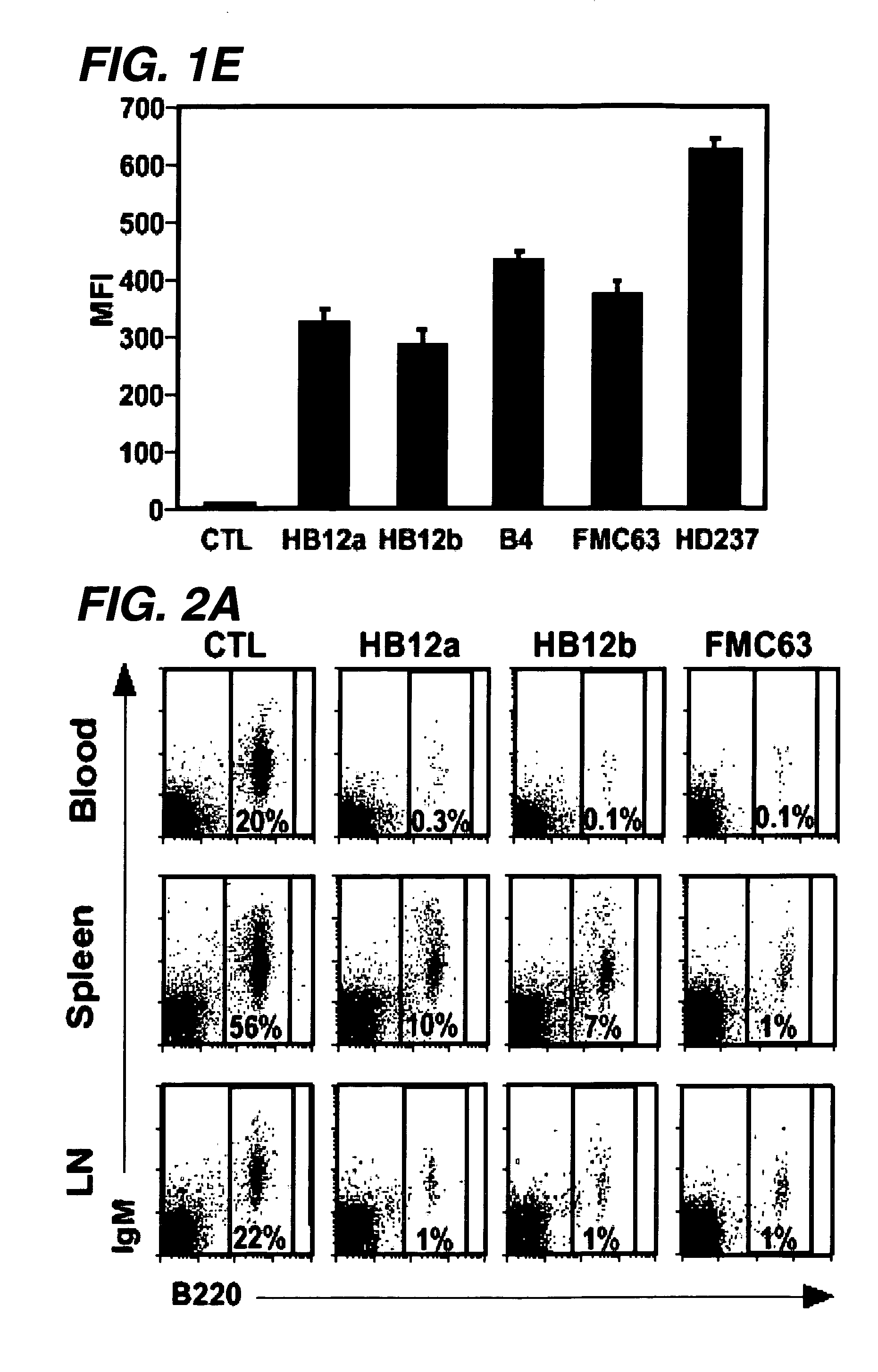
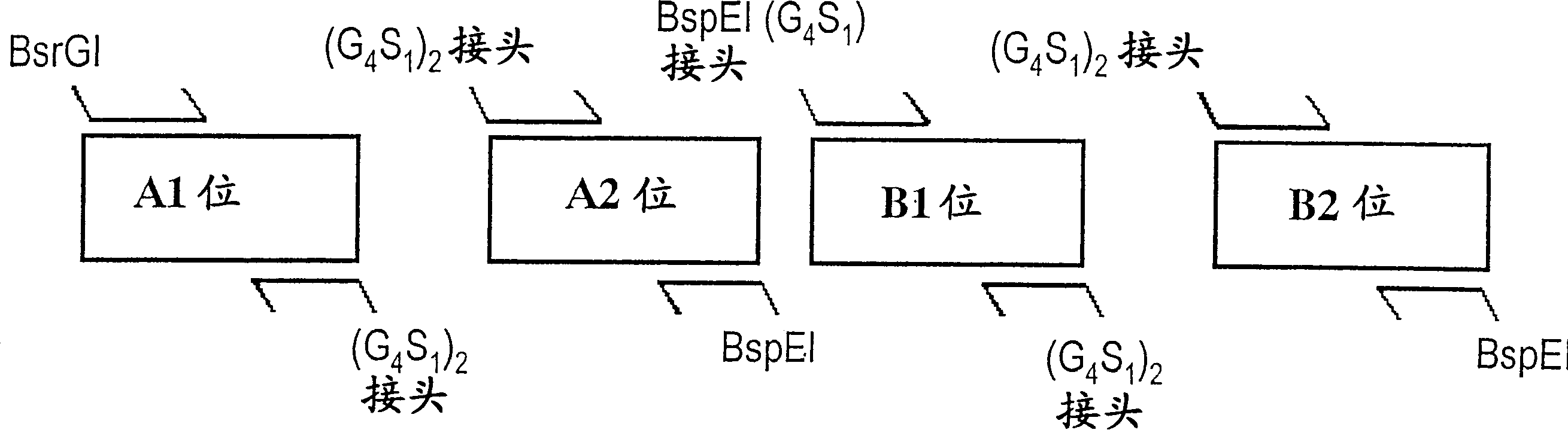
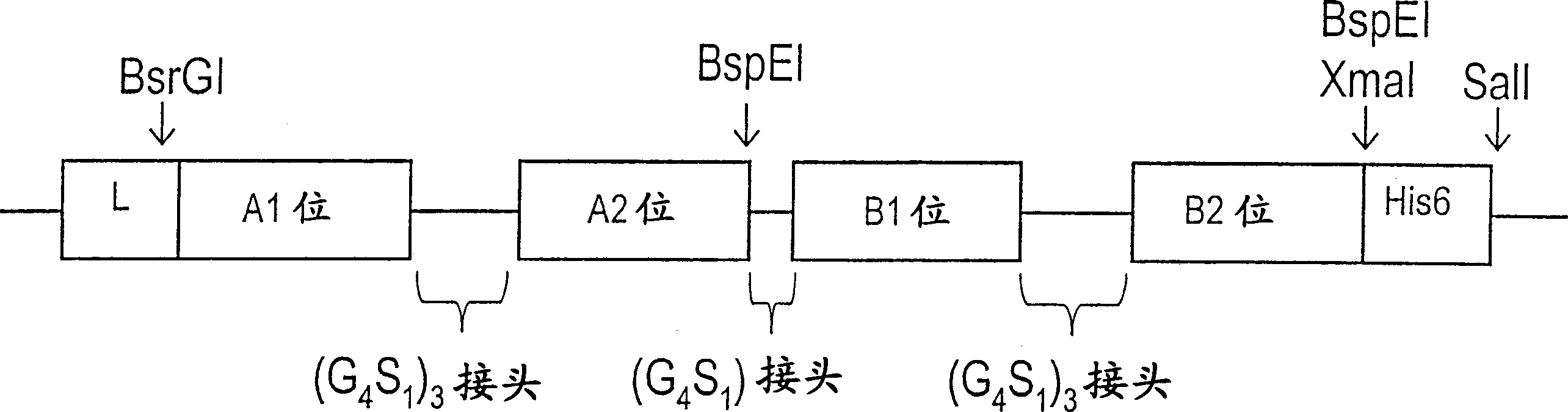
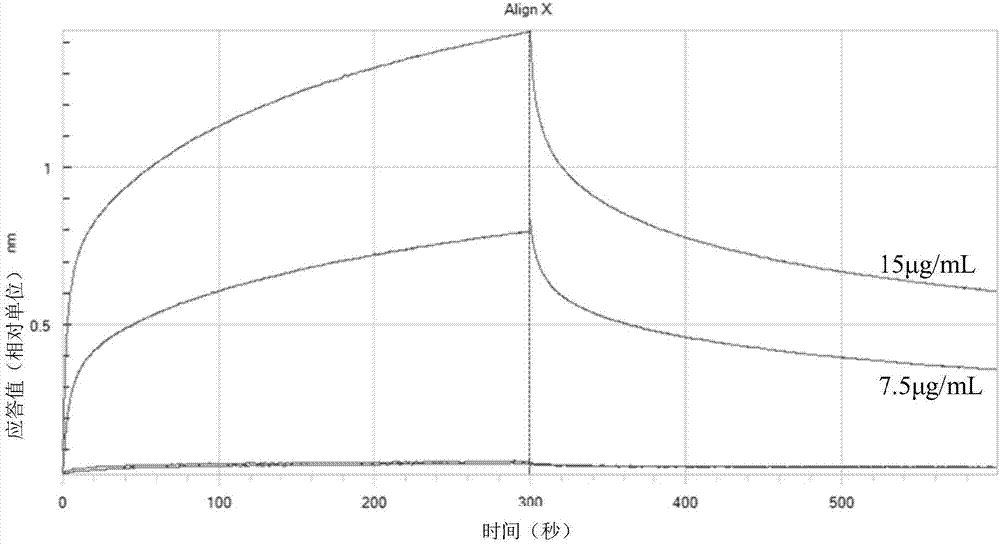
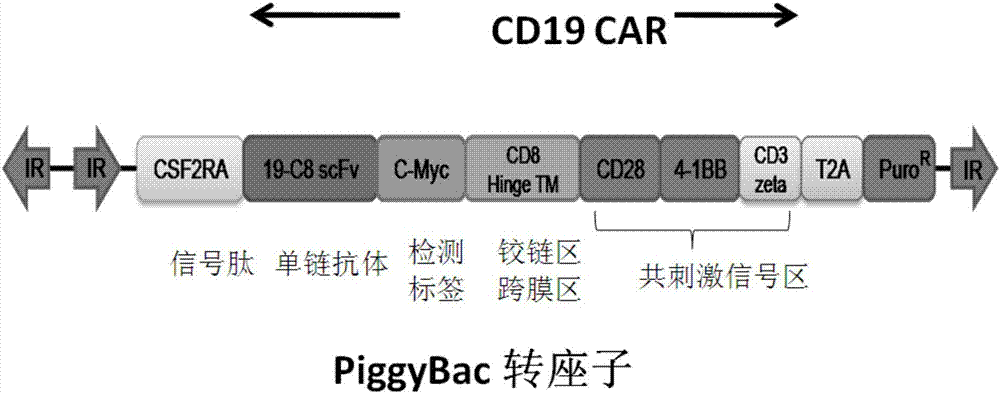
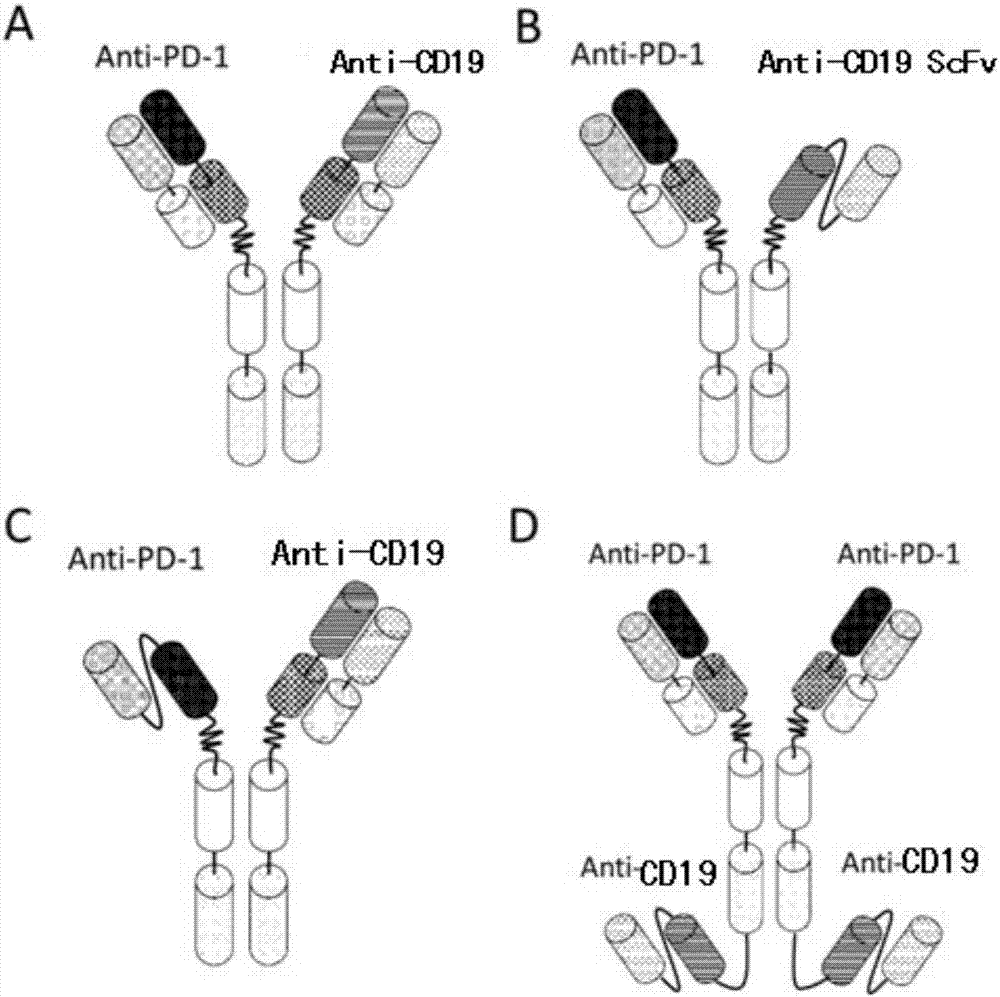
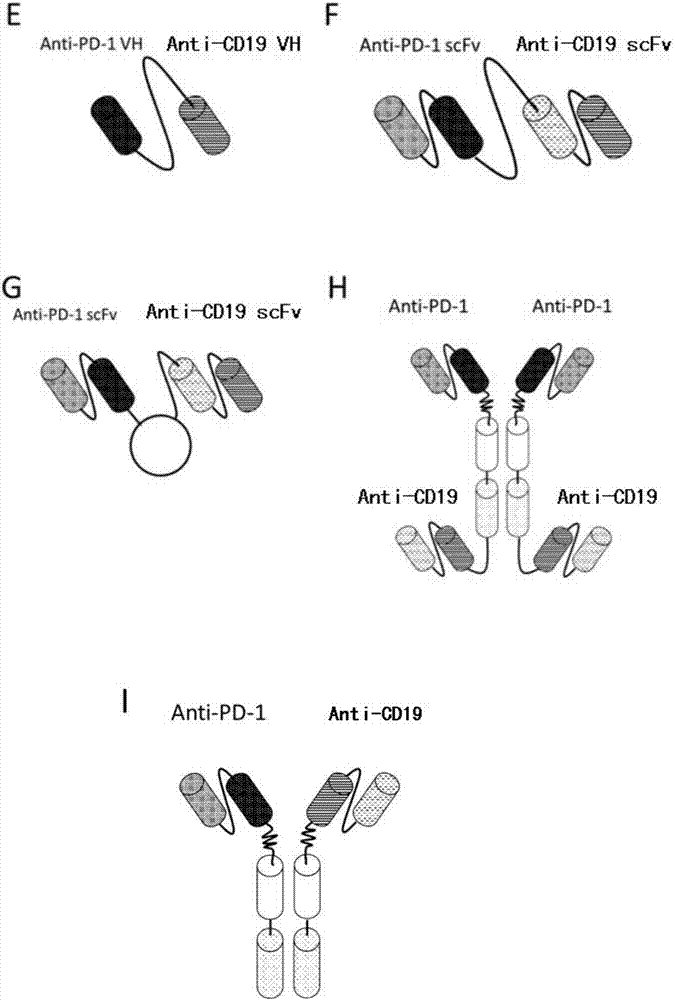
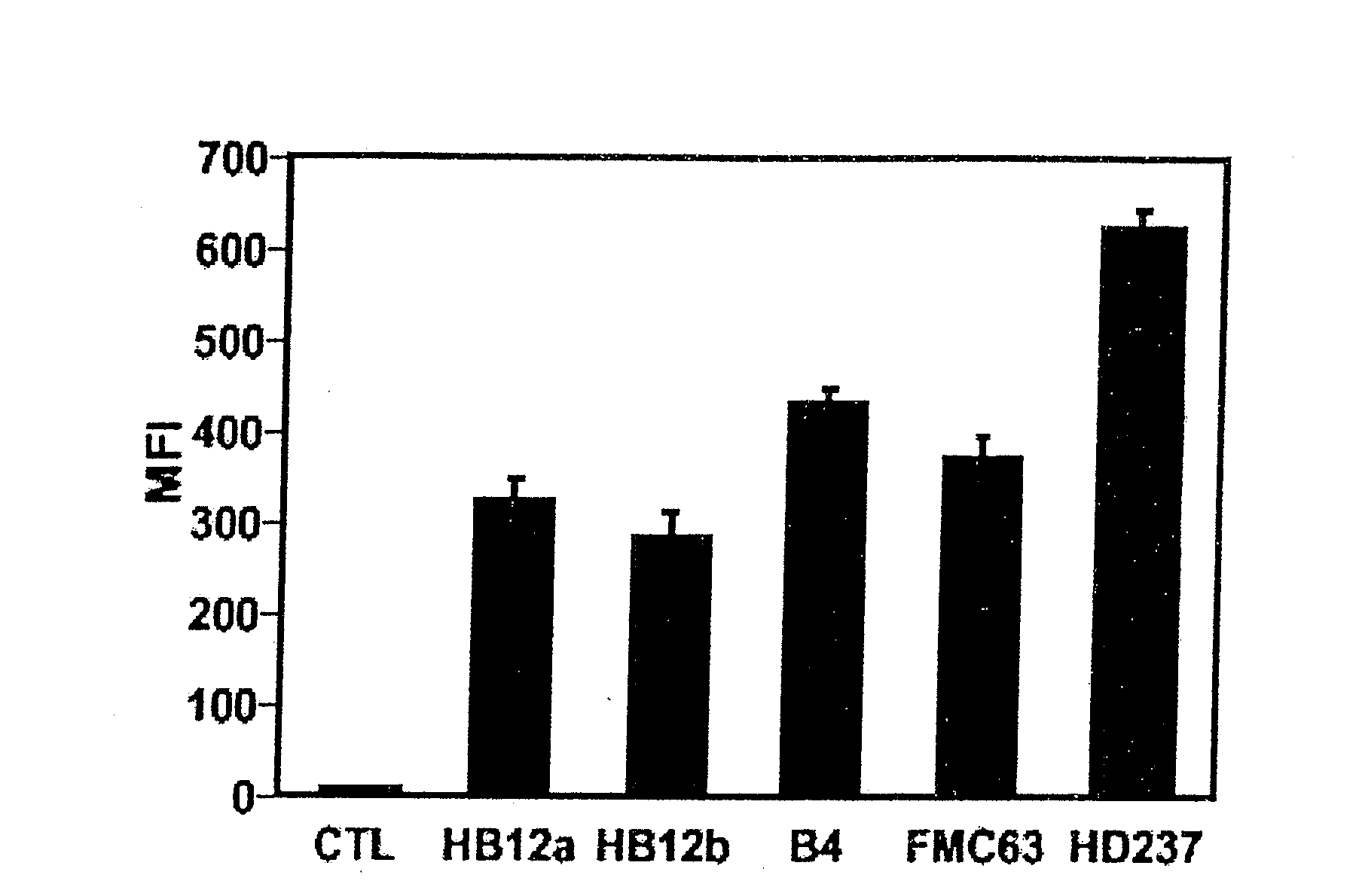
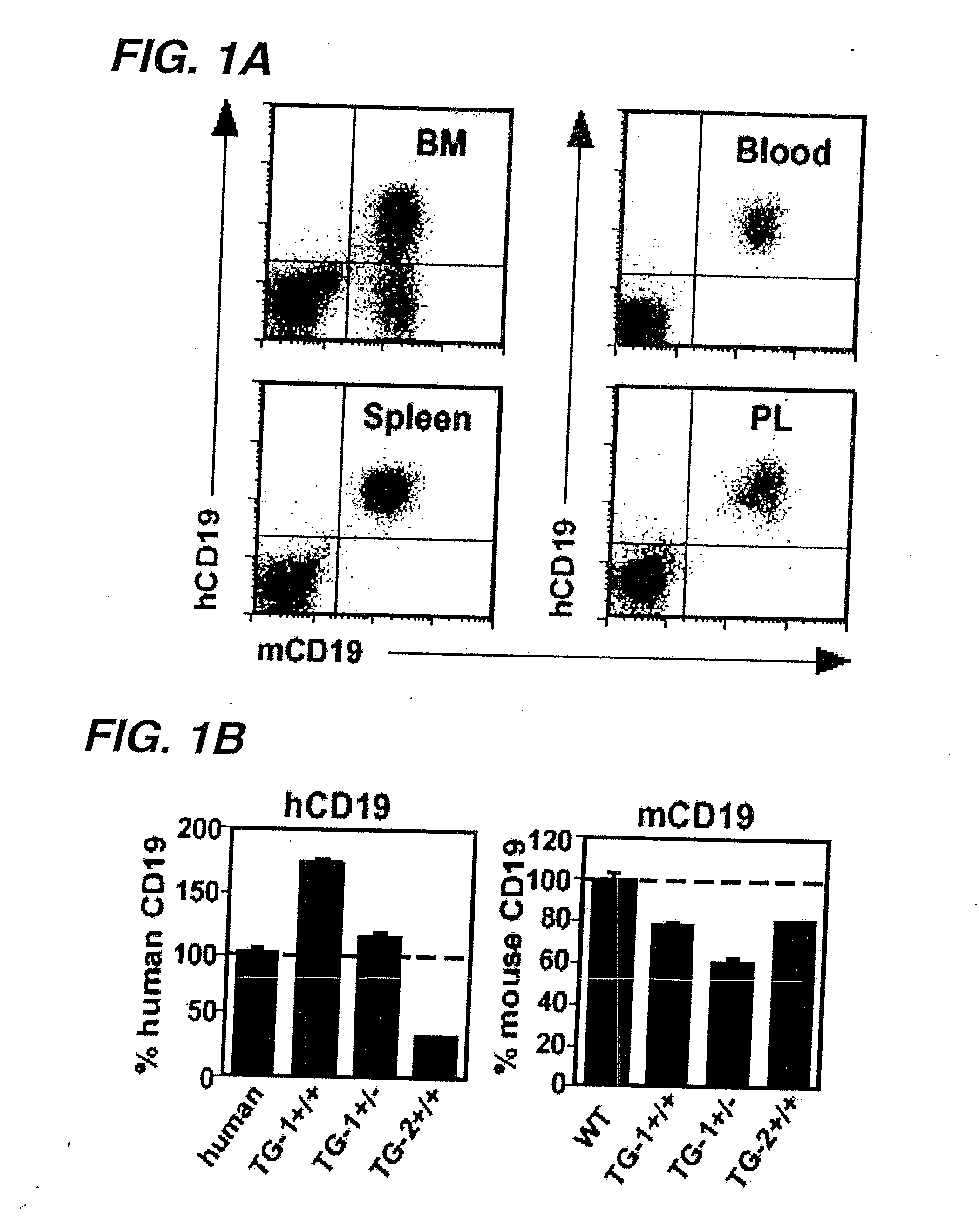
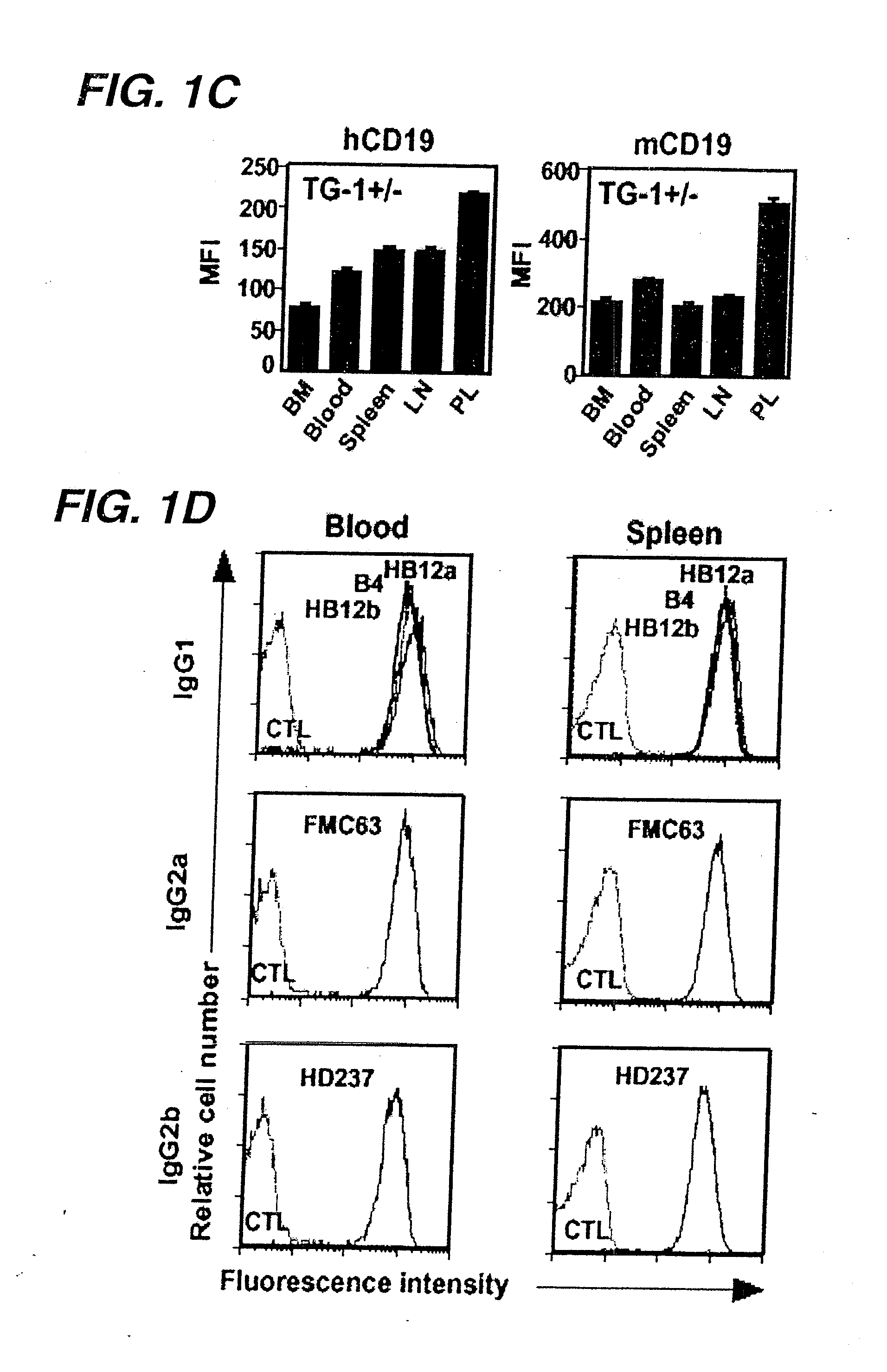
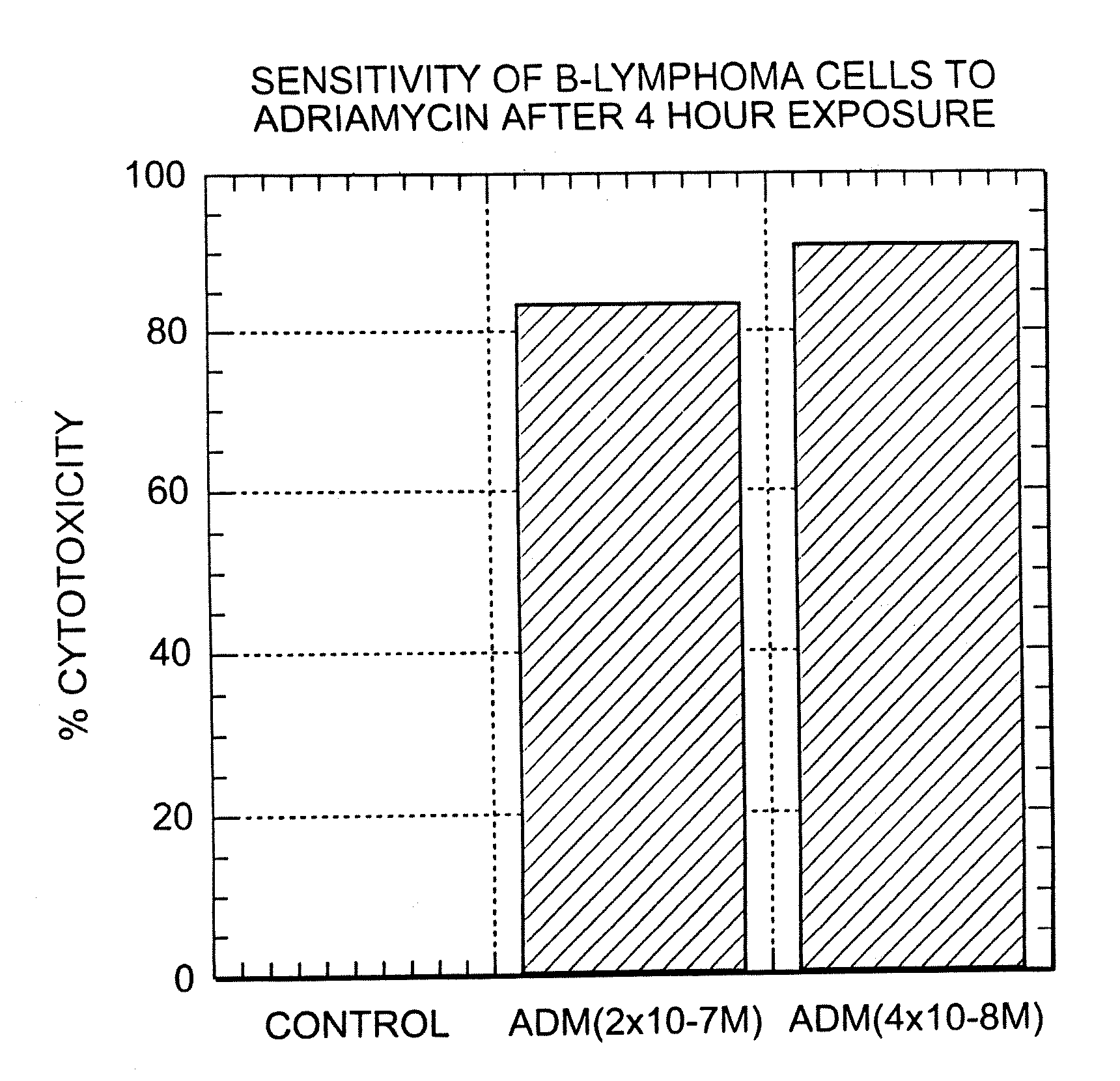
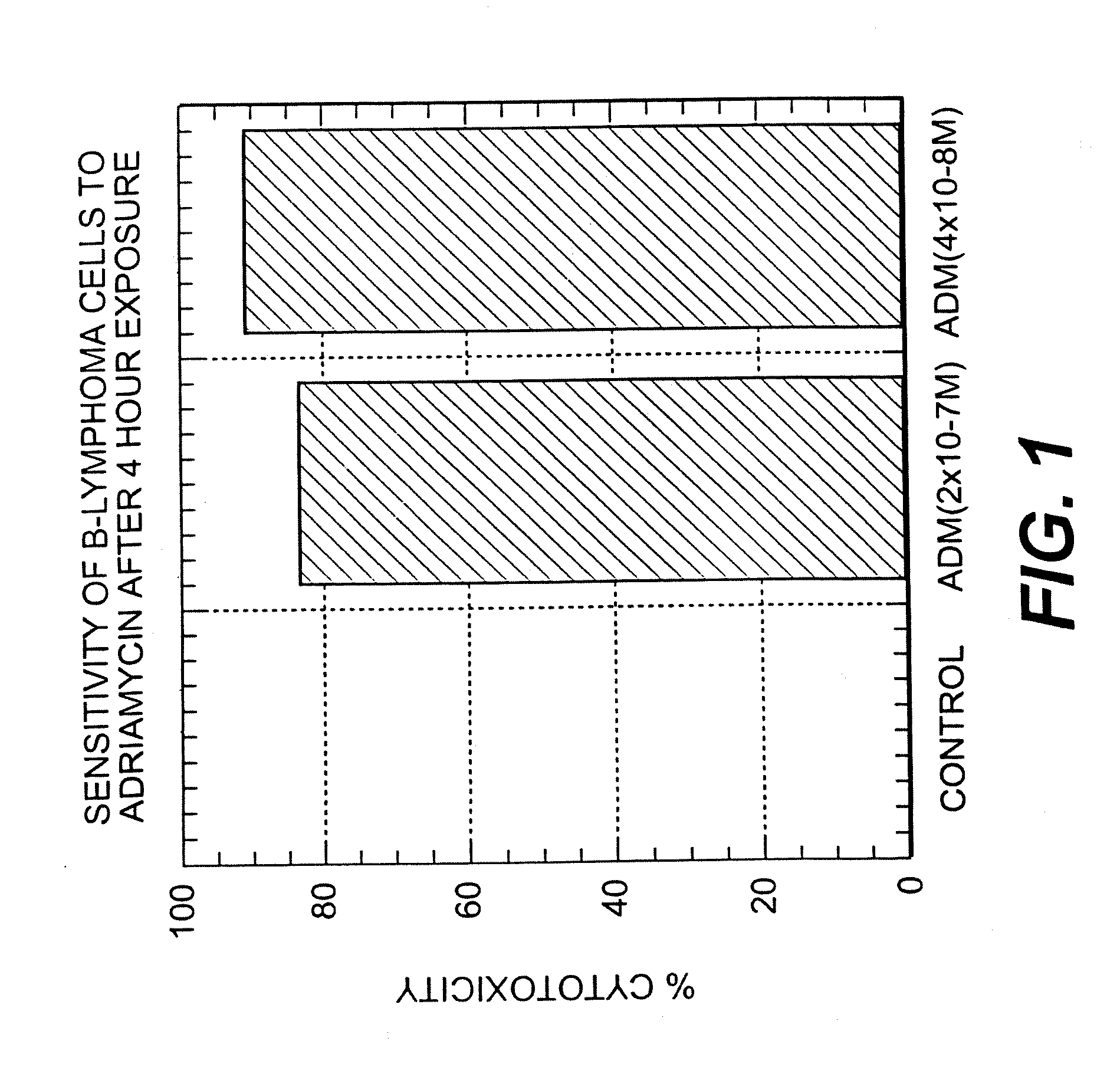
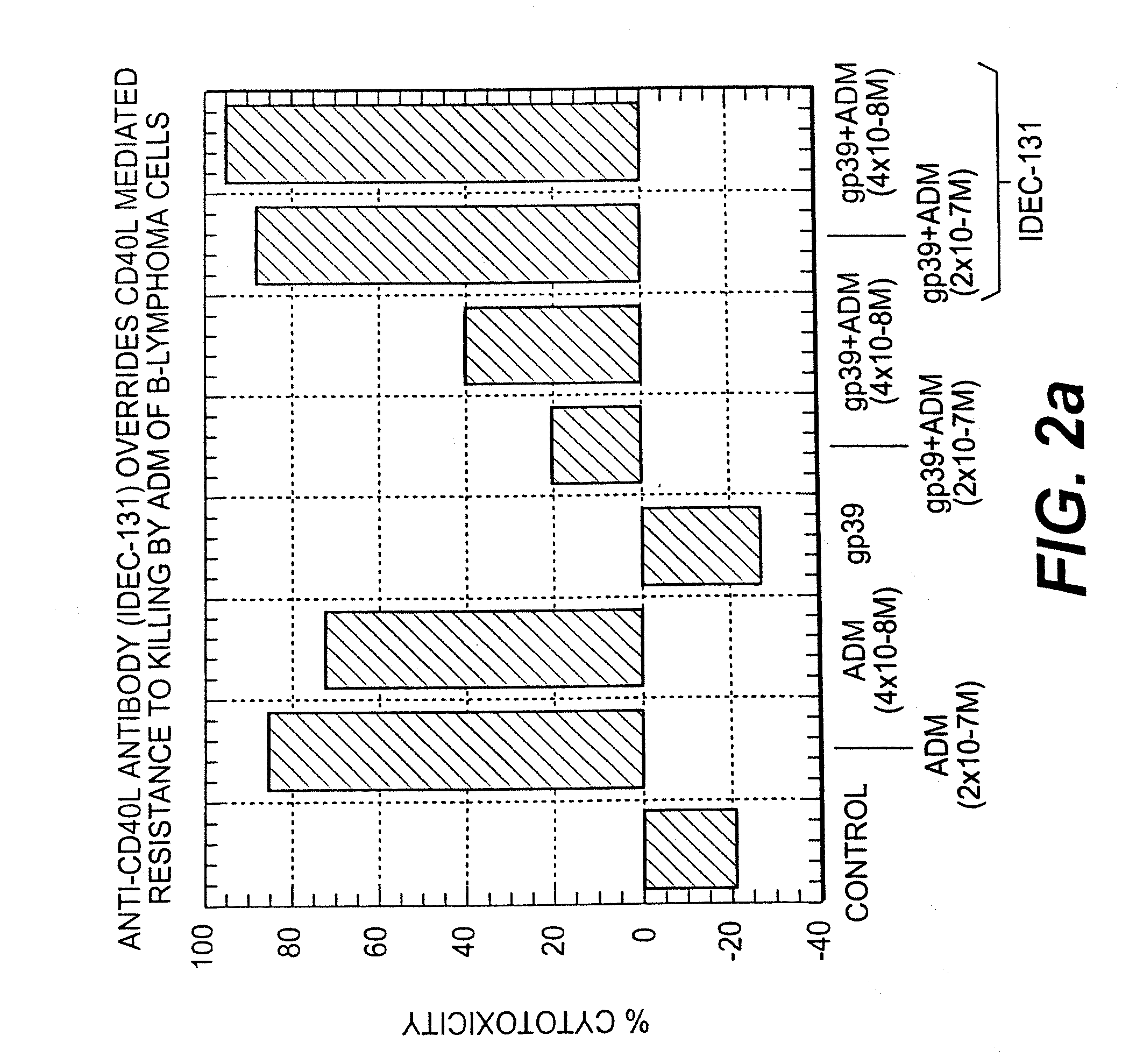
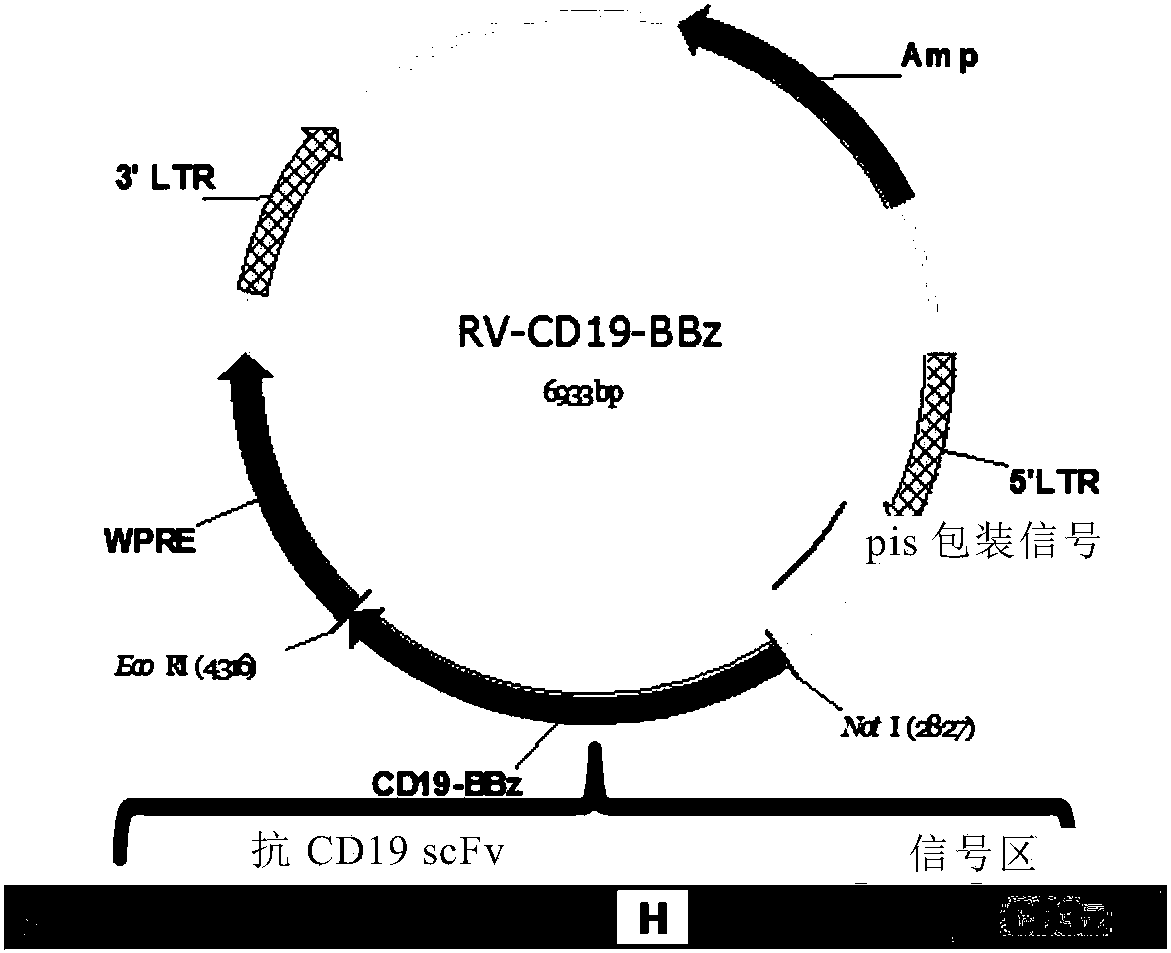
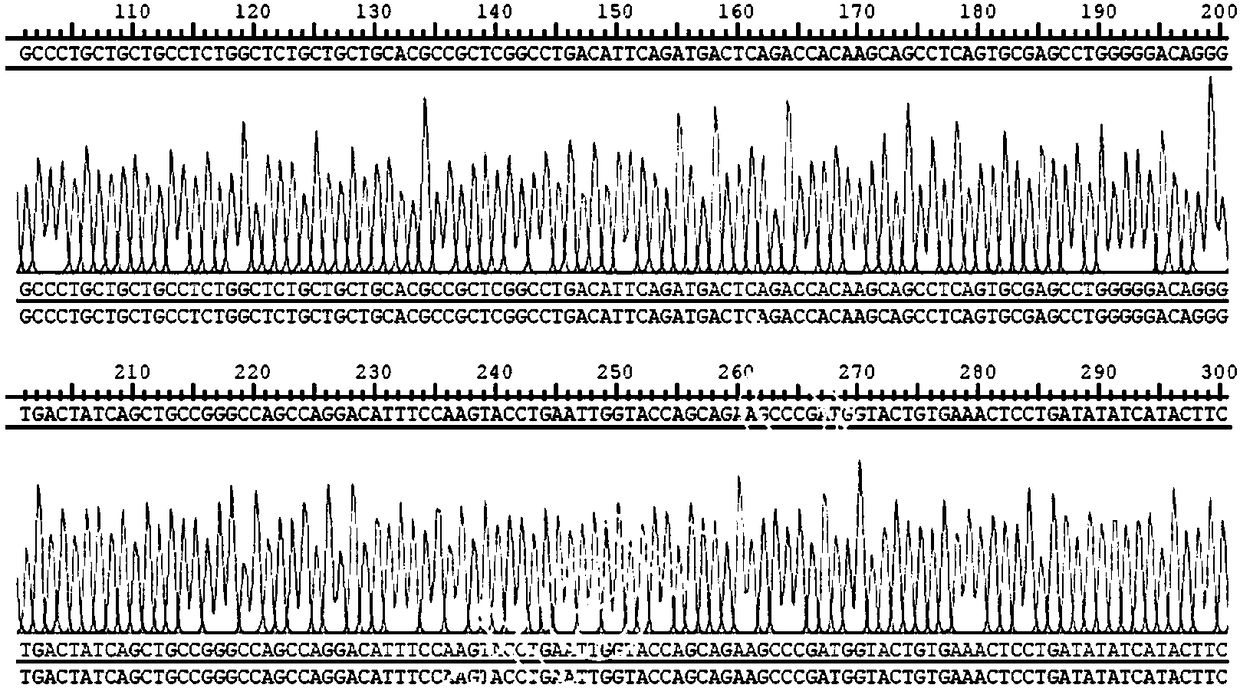
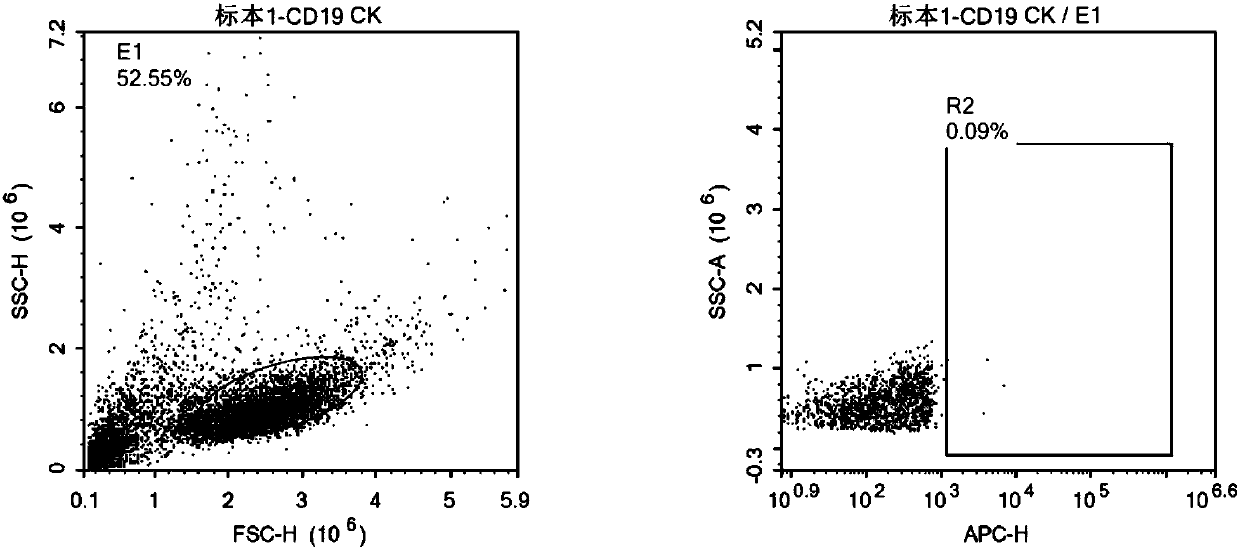
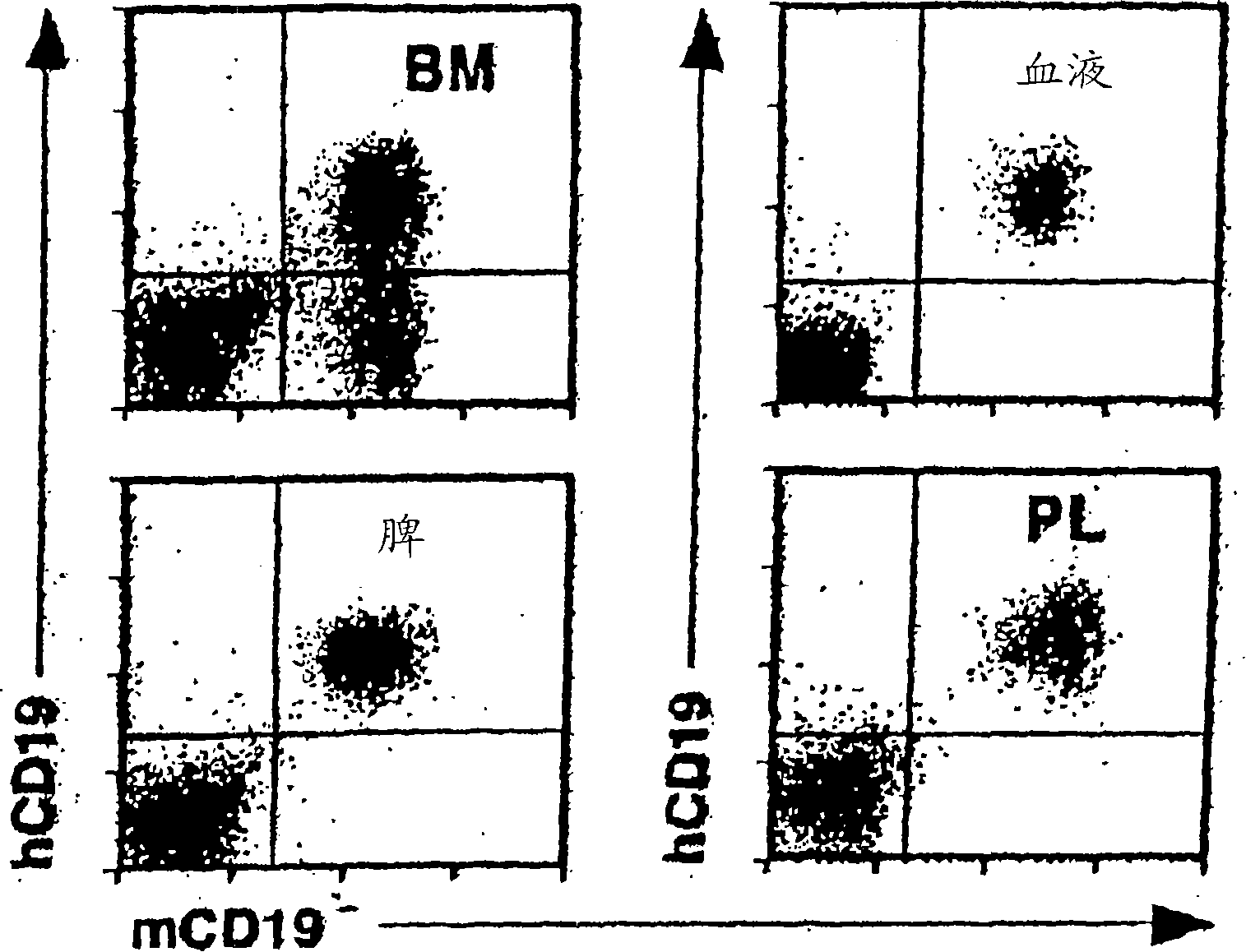
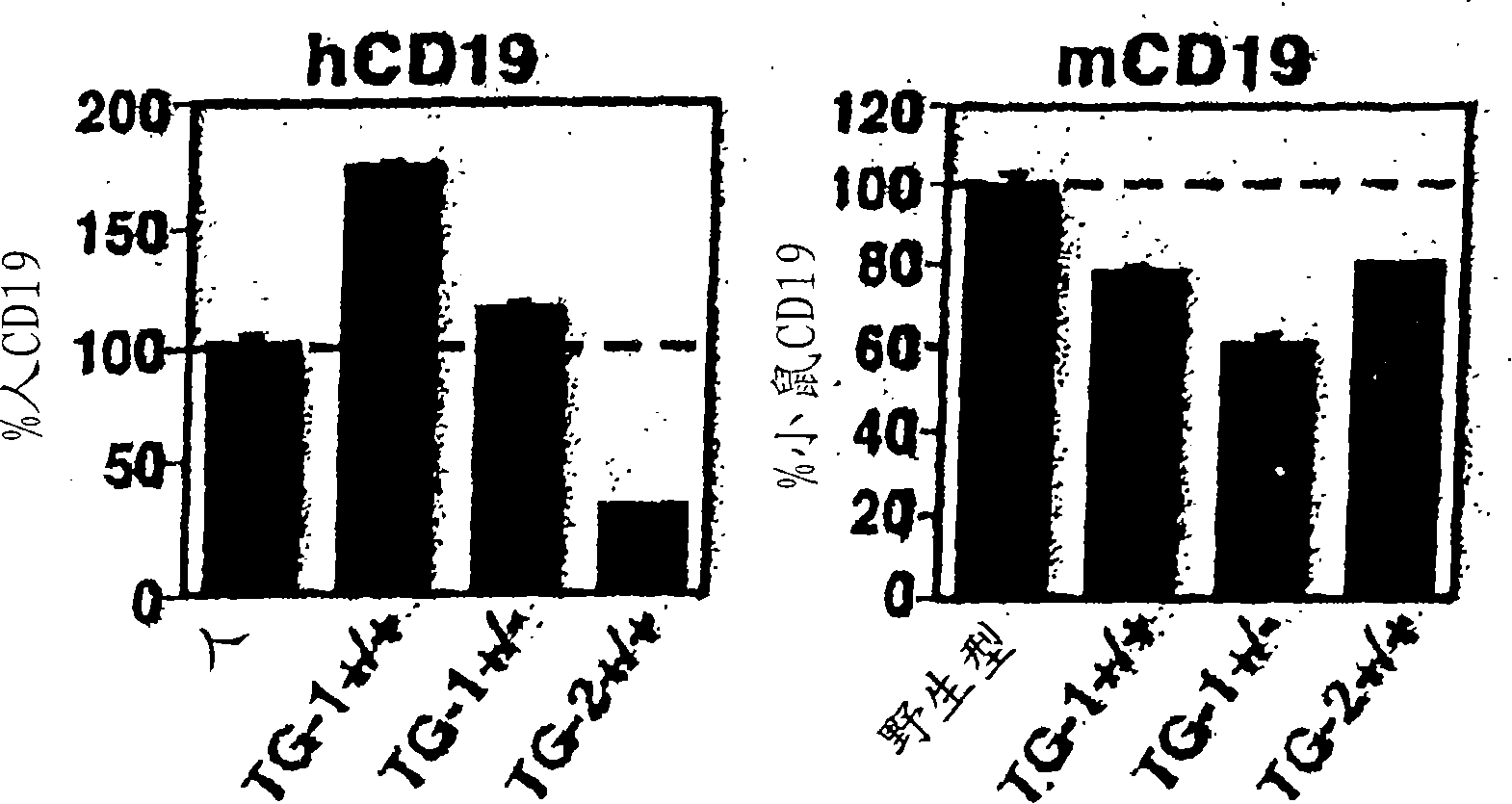
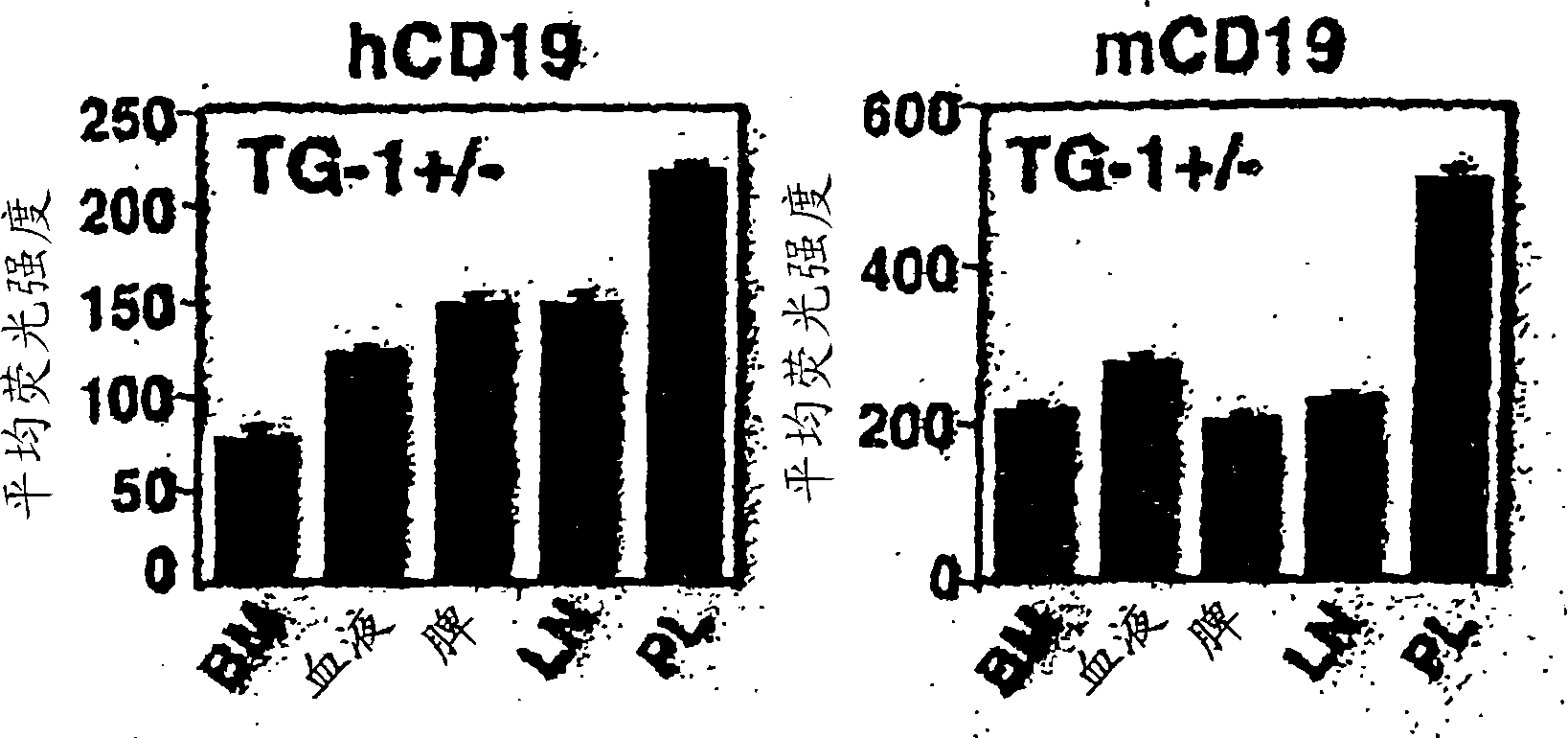
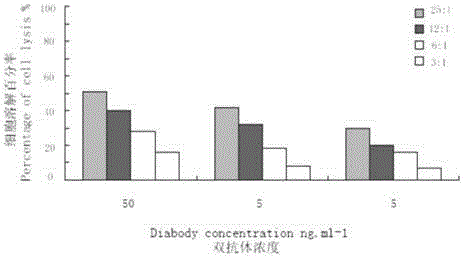
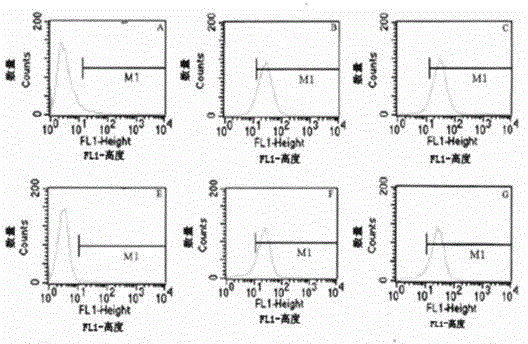
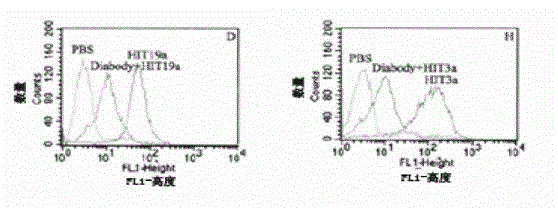
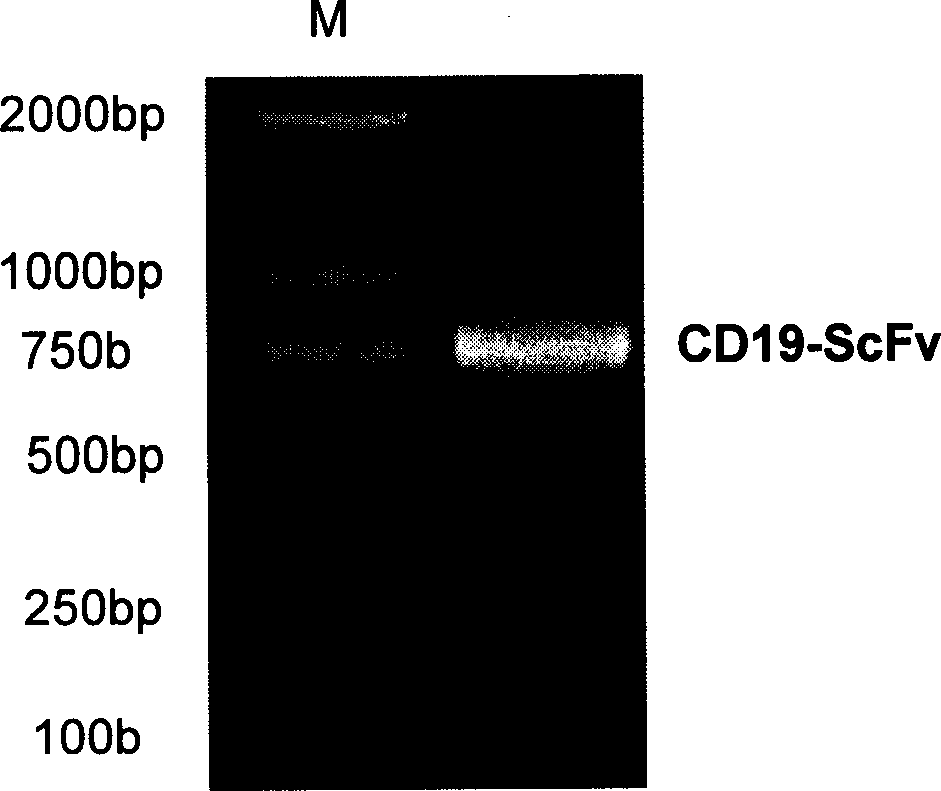
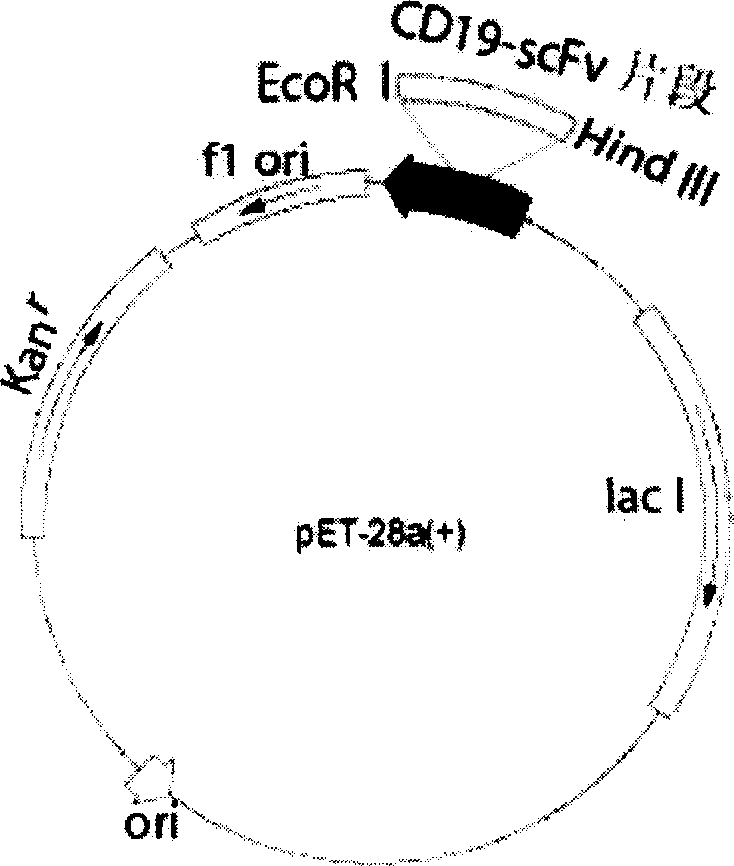
Patents
Literature
78 results about "Anti cd19" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immunoregulatory antibodies and uses thereof
InactiveUS20030103971A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCD37CD23
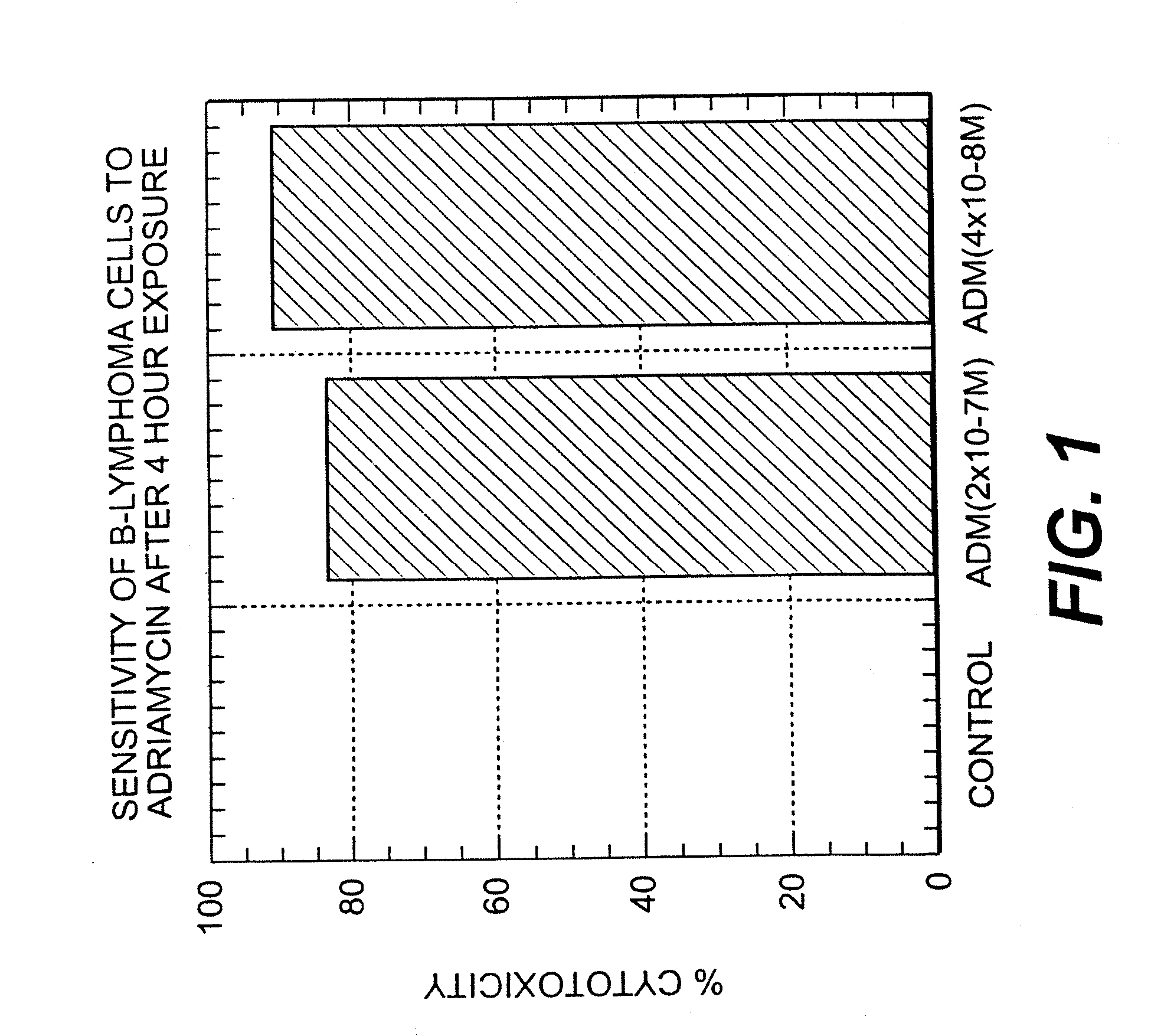
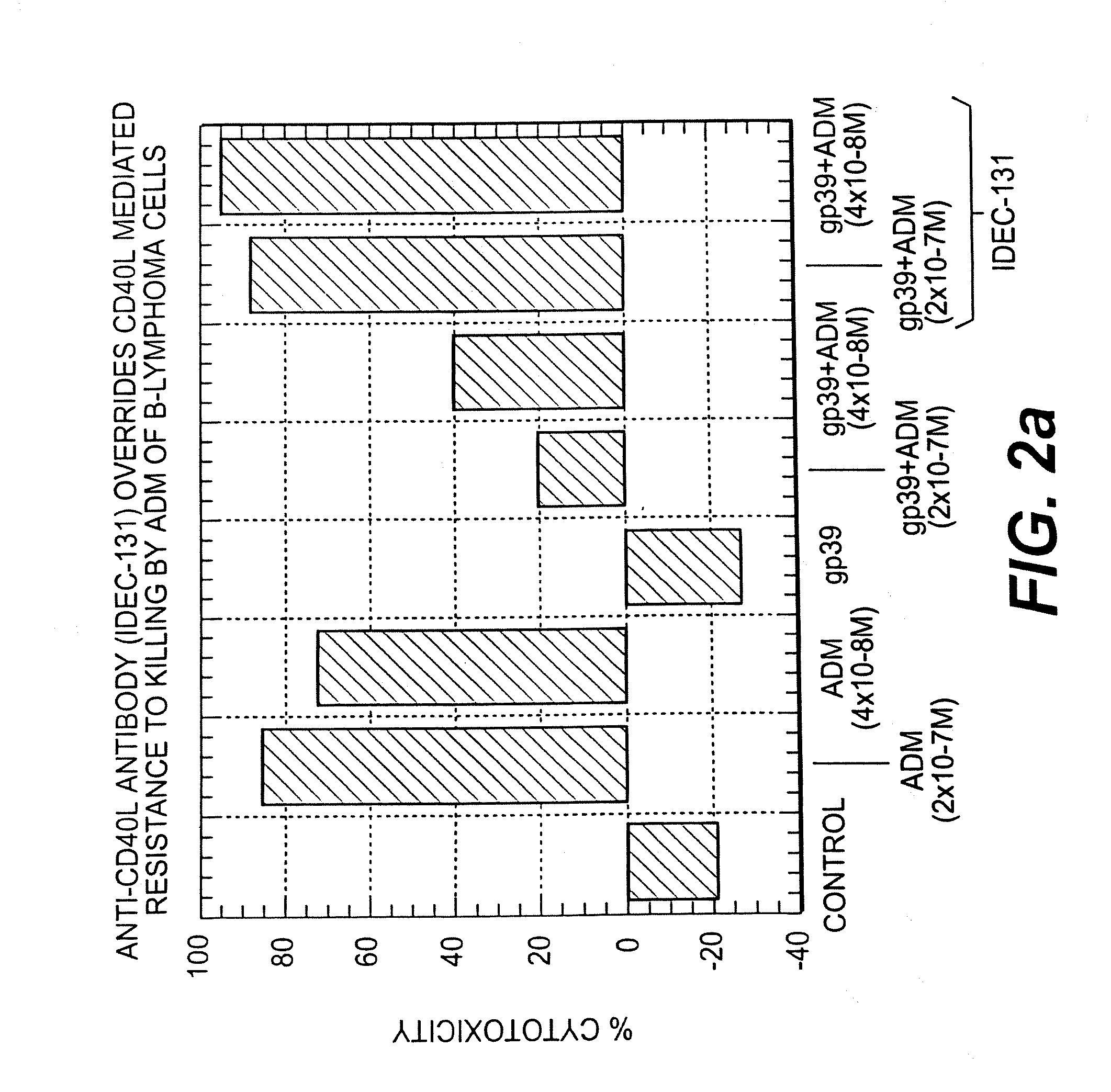
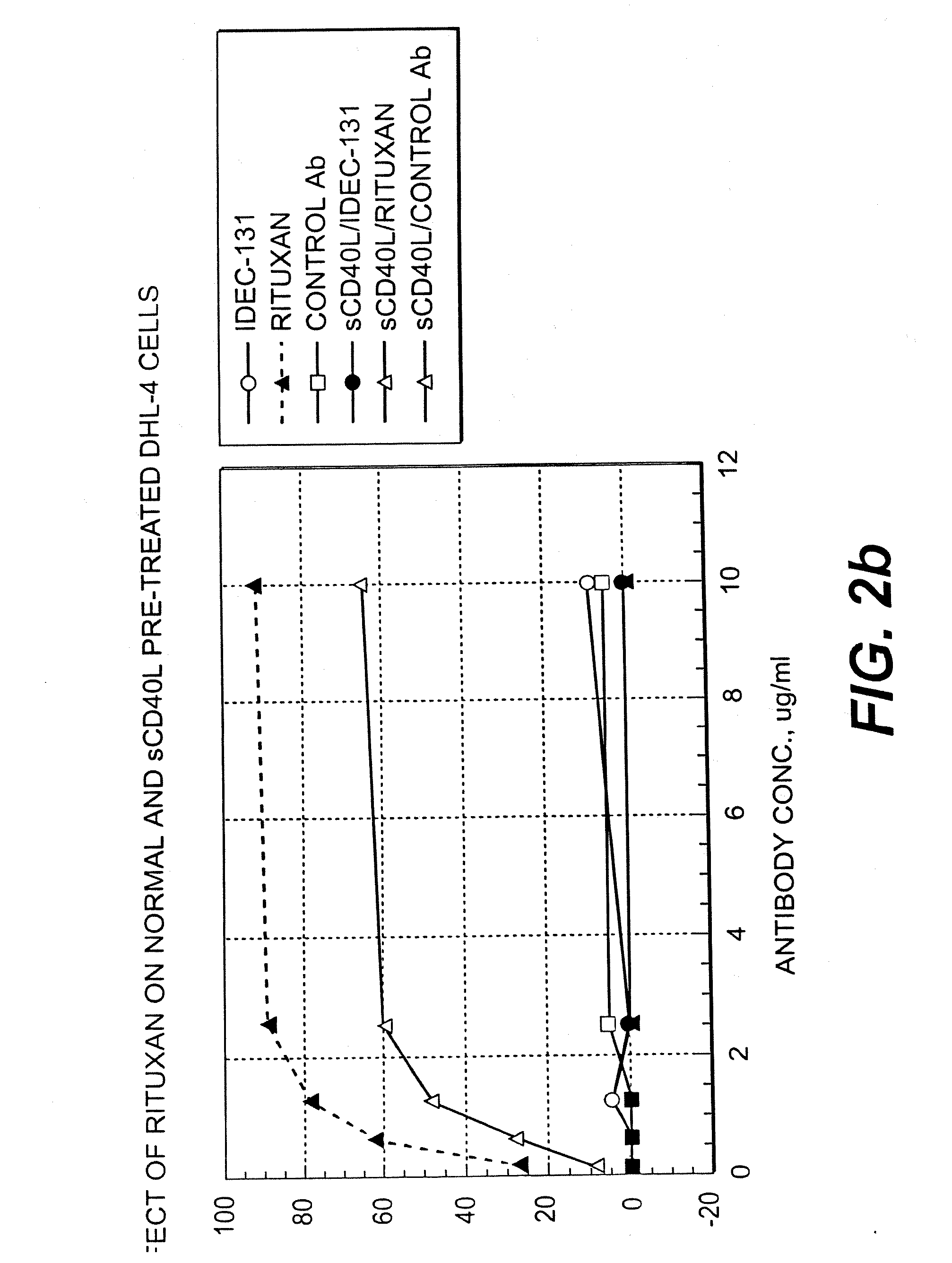
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:BIOGEN INC
Anti-CD19 antibodies and uses in oncology
InactiveUS20060233791A1Efficient productionEfficiently depletedAntibody ingredientsImmunoglobulinsDiseaseTherapeutic antibody
The invention relates to immunotherapeutic compositions and methods for the treatment of B cell diseases and disorders in human subjects, such as, but not limited to, B cell malignancies, using therapeutic antibodies that bind to the human CD19 antigen and that preferably mediate human ADCC. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD19 antibodies of the IgG1 or IgG3 human isotype. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD19 antibodies of the IgG2 or IgG4 human isotype that preferably mediate human ADCC. The present invention also relates to pharmaceutical compositions comprising chimerized anti-CD19 antibodies of the IgG1, IgG2, IgG3, or IgG4 isotype that mediate human ADCC. In preferred embodiments, the present invention relates to pharmaceutical compositions comprising monoclonal human, humanized, or chimeric anti-CD19 antibodies.
Owner:DUKE UNIV
Treatment of B cell malignancies using combination of B cell depleting antibody and immune modulating antibody related applications
InactiveUS20050123540A1Radioactive preparation carriersImmunoglobulins against cell receptors/antigens/surface-determinantsCD37Combination therapy
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:IDEC PHARM CORP +1
Anti-CD19 antibody therapy for transplantation
InactiveUS20060280738A1Efficient productionEfficiently depletedBiocidePhosphorous compound active ingredientsAntigenDisease
The invention relates to immunotherapeutic compositions and methods for the treatment and prevention of GVHD, humoral rejection, and post-transplantation lymphoproliferative disorder in human subjects using therapeutic antibodies that bind to the human CD19 antigen and that preferably mediate human ADCC. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD 19 antibodies of the IgG1 or IgG3 human isotype. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD19 antibodies of the IgG2 or IgG4 human isotype that preferably mediate human ADCC. The present invention also relates to pharmaceutical compositions comprising chimerized anti-CD19 antibodies of the IgG1, IgG2, IgG3, or IgG4 isotype that mediate human ADCC. In preferred embodiments, the present invention relates to pharmaceutical compositions comprising monoclonal human, humanized, or chimeric anti-CD19 antibodies.
Owner:DUKE UNIV
Anti-CD19 Antibodies
The present invention provides humanized, chimeric and human anti-CD19 antibodies, anti-CD19 antibody fusion proteins, and fragments thereof that bind to a human B cell marker. Such antibodies, fusion proteins and fragments thereof are useful for the treatment and diagnosis of various B-cell disorders, including B-cell malignancies and autoimmune diseases. In more particular embodiments, the humanized anti-CD19 antibodies may comprise one or more framework region amino acid substitutions designed to improve protein stability, antibody binding and / or expression levels. In a particularly preferred embodiment, the substitutions comprise a Ser91Phe substitution in the hA19 VH sequence.
Owner:IMMUNOMEDICS INC
CD19-specific immunotoxin and treatment method
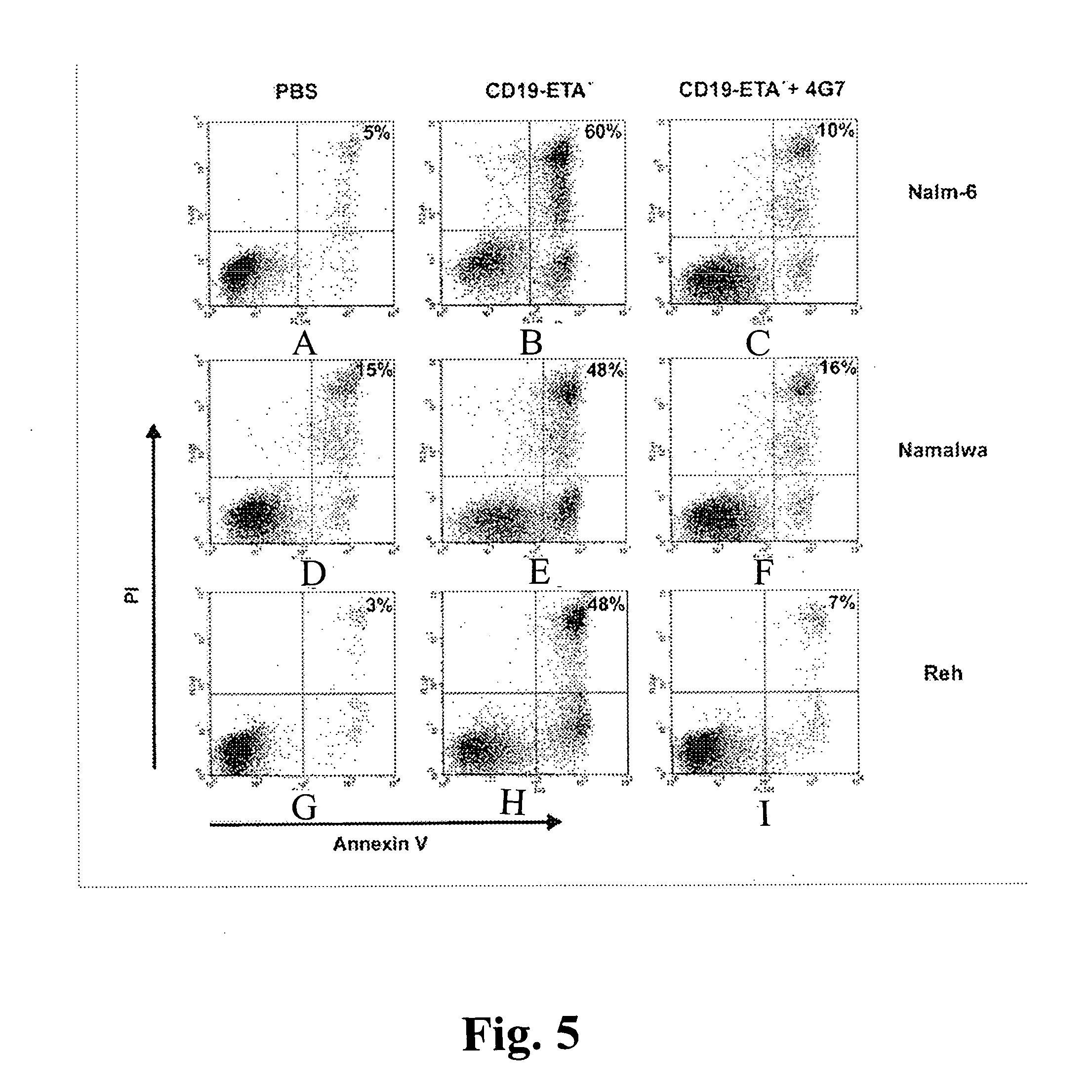
InactiveUS20070178103A1Easy to transportResistant to extracellular cleavagePolypeptide with localisation/targeting motifHybrid immunoglobulinsAutoimmune conditionBacterial exotoxin
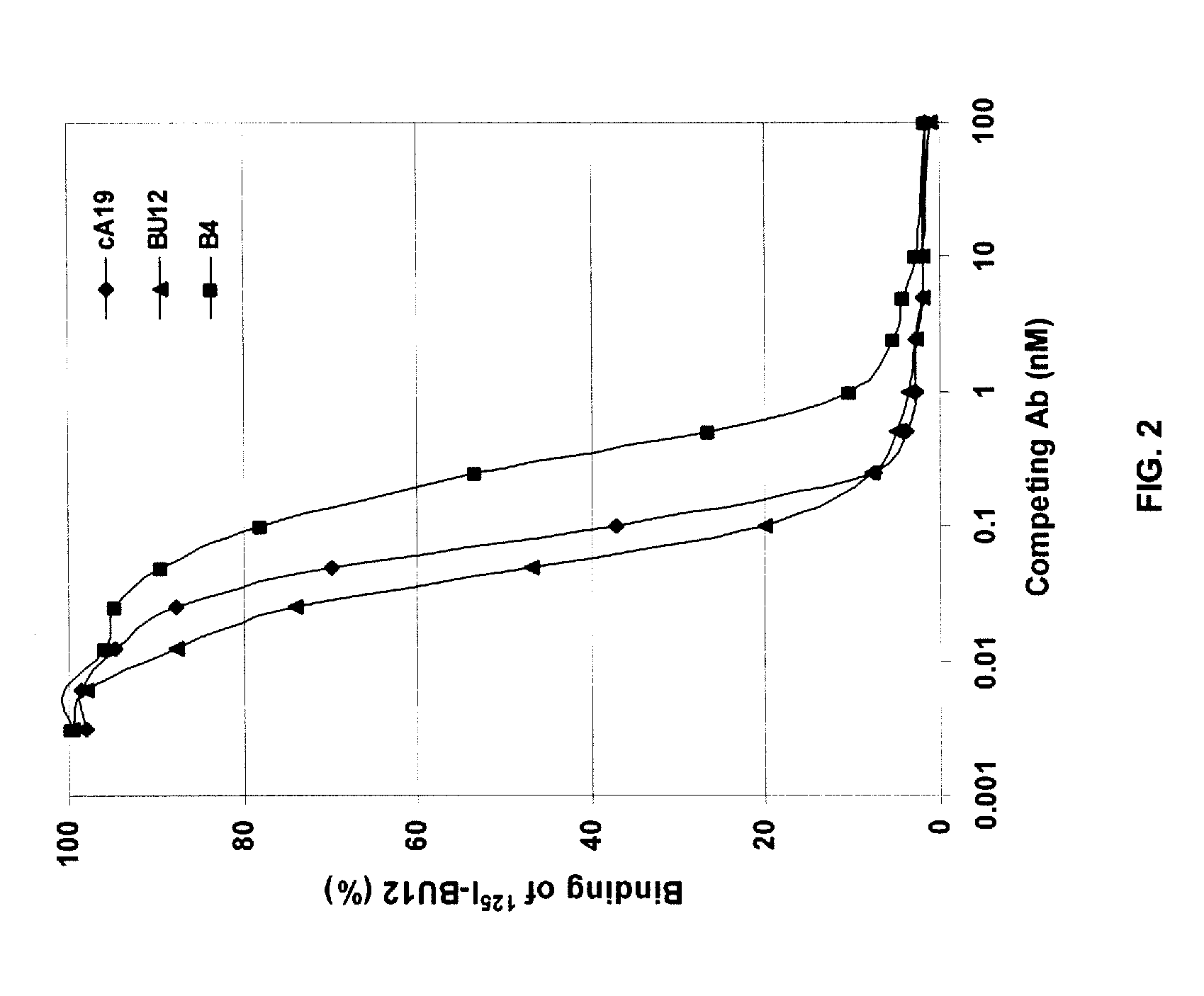
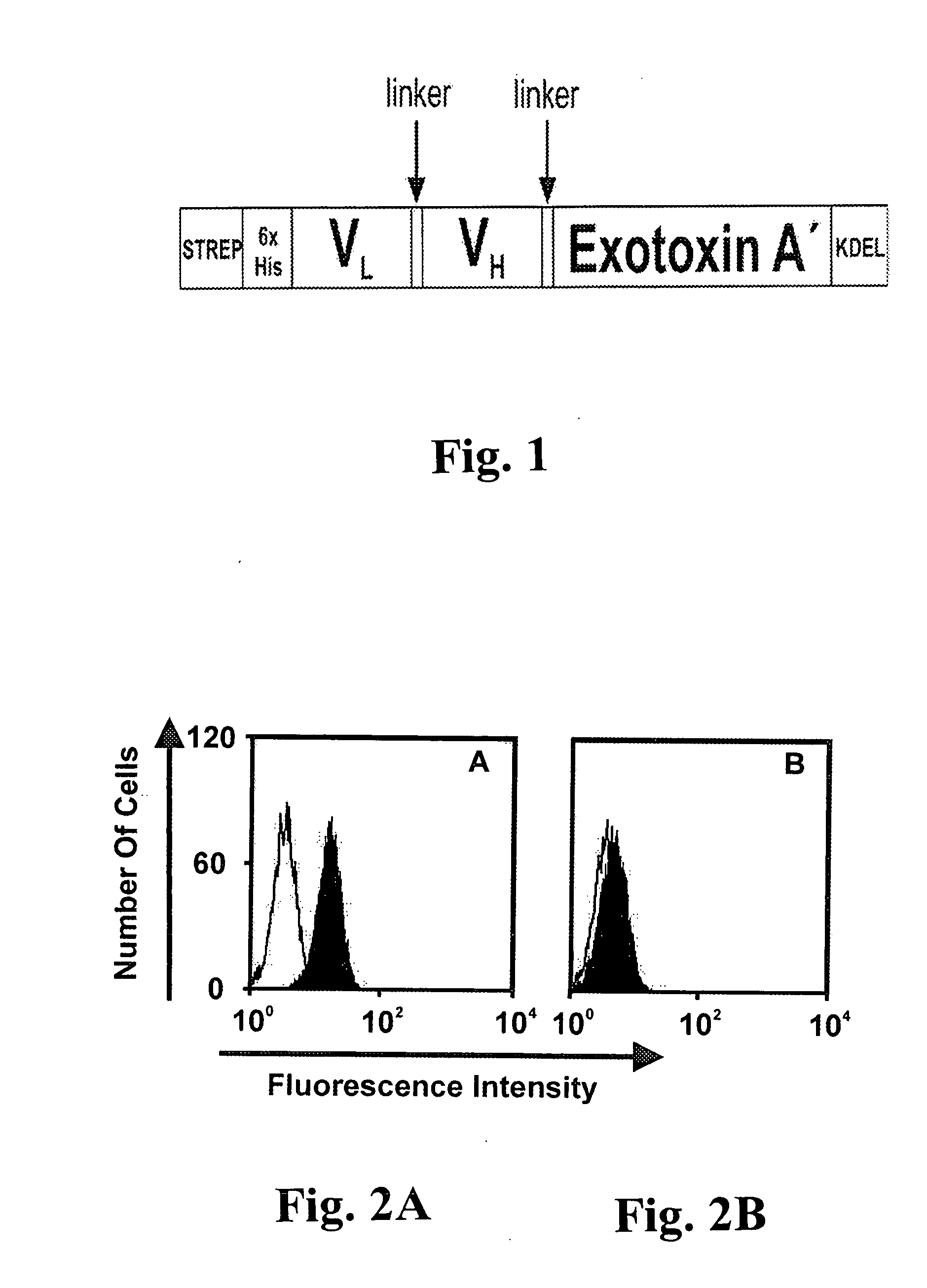
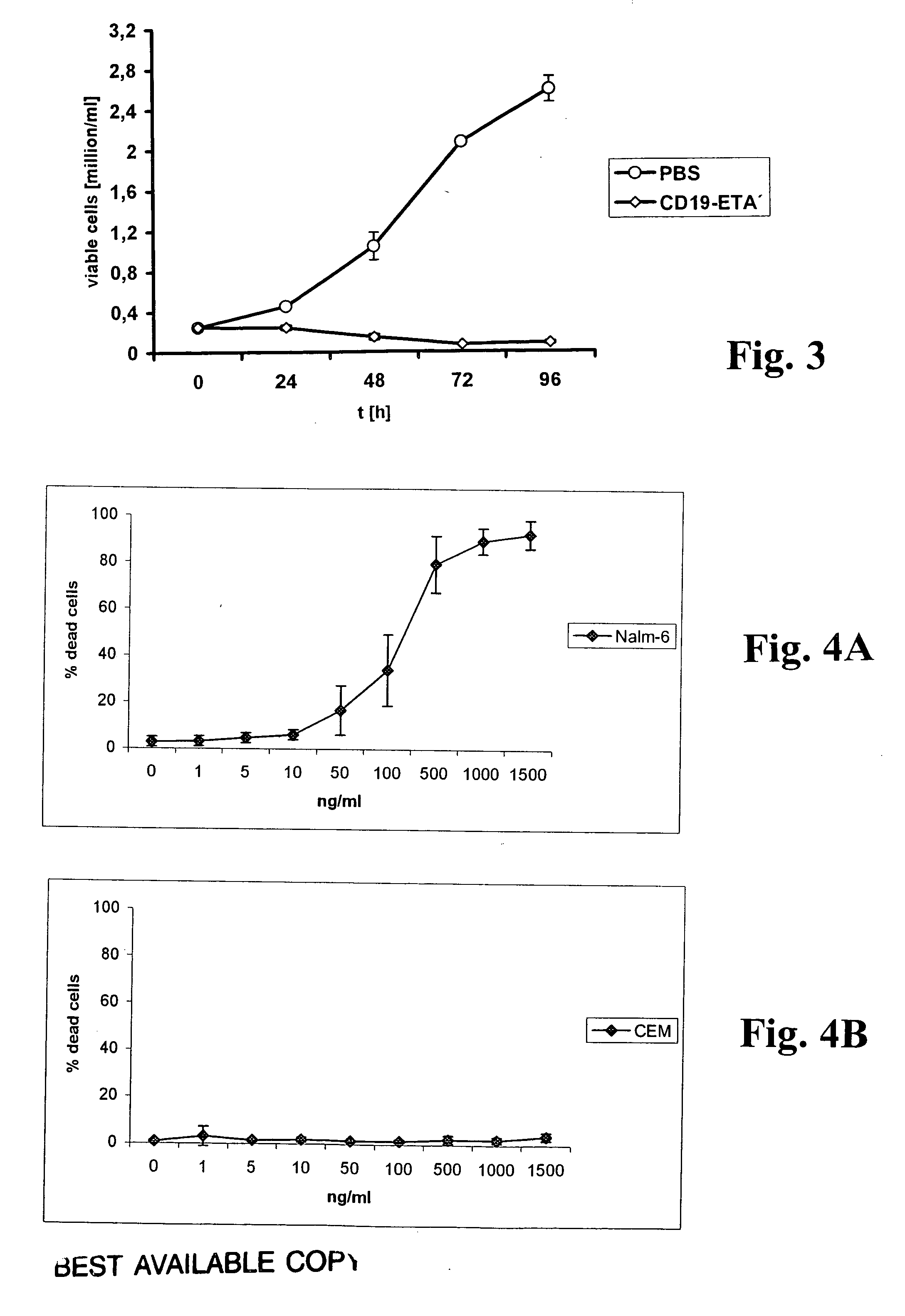
An immunotoxin for use in, and a method for treating a subject having a cancer associated with malignant B-lineage cells or an autoimmune condition, are disclosed. The immunotoxin includes (a) an anti-CD19 antibody lacking an Fc fragment, (b) a modified exotoxin A protein having both Domains II and III, but lacking Domain I, and (c) a peptide linker joining the C-terminal end of the antibody to the N-terminal end of the modified exotoxin A protein. The linker is substantially resistant to extracellular cleavage. The modified exotoxin A protein may be further modified to include a C-terminal KDEL sequence (SEQ ID NO: 6) that promotes transport of the protein to the endoplasmic reticulum of cells that have taken up the immunotoxin.
Owner:FRIEDRICH ALEXANDER UNIV ERLANGEN NURNBERG
Immunoregulatory Antibodies and Uses Thereof
InactiveUS20070009519A1Convenient treatmentIn-vivo radioactive preparationsPharmaceutical containersCD37Combination therapy
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:BIOGEN INC
Anti-CD19 scFv (FMC63) polypeptide
ActiveUS9701758B2Polypeptide with localisation/targeting motifImmunoglobulin superfamilyMonoclonal antibodyBiological activation
Provided are monoclonal antibodies that detect CD 19 CAR-modified immune cells and CAR-modified immune cells irrespective of the tumor associated antigen they target. Methods of using these functional monoclonal antibodies include, but are not limited to, detection, quantification, activation, and selective propagation of CAR-modified immune cells.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Anti-CD19 antibody therapy for autoimmune disease
InactiveUS20060263357A1Efficient productionEfficiently depletedAntipyreticMetabolism disorderAntigenImmunologic disorders
The invention relates to immunotherapeutic compositions and methods for the treatment of autoimmune diseases and disorders in human subjects using therapeutic antibodies that bind to the human CD19 antigen and that preferably mediate human ADCC. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD19 antibodies of the IgG1 or IgG3 human isotype. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD19 antibodies of the IgG2 or IgG4 human isotype that preferably mediate human ADCC. The present invention also relates to pharmaceutical compositions comprising chimerized anti-CD19 antibodies of the IgG1, IgG2, IgG3, or IgG4 isotype that mediate human ADCC. In preferred embodiments, the present invention relates to pharmaceutical compositions comprising monoclonal human, humanized, or chimeric anti-CD19 antibodies.
Owner:DUKE UNIV
Pharmaceutical compositions comprising bispecific anti-cd3, anti-cd19 antibody constructs for the treatment of b-cell related disorders
The present invention relates to a pharmaceutical composition comprising a bispecific single chain antibody construct comprising human CD3 and human CD19 specific binding domains, wherein the corresponding variable heavy chain regions (VH) and the corresponding variable light chain region (VL) from N-terminus to C-terminus in the following order: VH (CD19)-VL (CD19)-VH (CD3)-VL (CD3), VH (CD3)-VL (CD3)-VH (CD19)-VL (CD19) or VH (CD3)-VL (CD3)-VL (CD19)-VH (CD19). In addition, the present invention discloses the preparation method of the pharmaceutical composition and the medical / pharmaceutical use of the specific bispecific single chain antibody molecule specific to human CD3 antigen and human CD19 antigen.
Owner:AMGEN RES (MUNICH) GMBH
Treatment of cancer using humanized Anti-cd19 chimeric antigen receptor
ActiveUS20190135940A1Good curative effectPolypeptide with localisation/targeting motifImmunoglobulin superfamilyBinding domainCancer research
The invention provides compositions and methods for treating diseases associated with expression of CD19. The invention also relates to chimeric antigen receptor (CAR) specific to CD19, vectors encoding the same, and recombinant T cells comprising the CD19 CAR. The invention also includes methods of administering a genetically modified T cell expressing a CAR that comprises a CD19 binding domain.
Owner:NOVARTIS AG +1
Immunoconjugates with improved efficacy for the treatment of diseases
InactiveUS20070196274A1Improve therapeutic efficacyHigh detection sensitivityIn-vivo radioactive preparationsImmunoglobulins against animals/humansAntibody conjugateCD11a
The invention provides therapeutic or diagnostic antibodies with modified N— or C-terminal sequences that are enriched with lysine or tyrosine residues. These lysine or tyfosine residues can be used to couple radioisotopes, cytotoxic agents, or detectable labels. The increased stoichiometric ratios of these agents in the antibody conjugates lead to improved therapeutic efficacy or enhanced detection sensitivity. Non-limiting examples of antibodies suitable for the present invention include anti-CD22, anti-ErbB2, anti-VEGF, anti-EGFR, anti-VEGFR, anti-Her-3, anti-Her-4, anti-CEA, anti-CTLA-4, anti-CD4, anti-CD3, anti-CD20, anti-TNF-a, anti-CD11a, anti-Lewis Y antigen, anti-TrailR, anti-IL2R, anti-CD30, anti-CD146, anti-CD147, anti-alpha V integrin beta, anti-CD19, anti-GD2, anti-3H11, anti-EBV, anti-HIV, anti-HBV, anti-HCV, and other disease-specific antibodies.
Owner:WELSON PHARMA
Target CD19 and CD22 chimeric antigen receptor and application thereof
PendingCN108504668AShorten the timeStable expressionMammal material medical ingredientsImmunoglobulinsSingle-Chain AntibodiesAntigen receptors
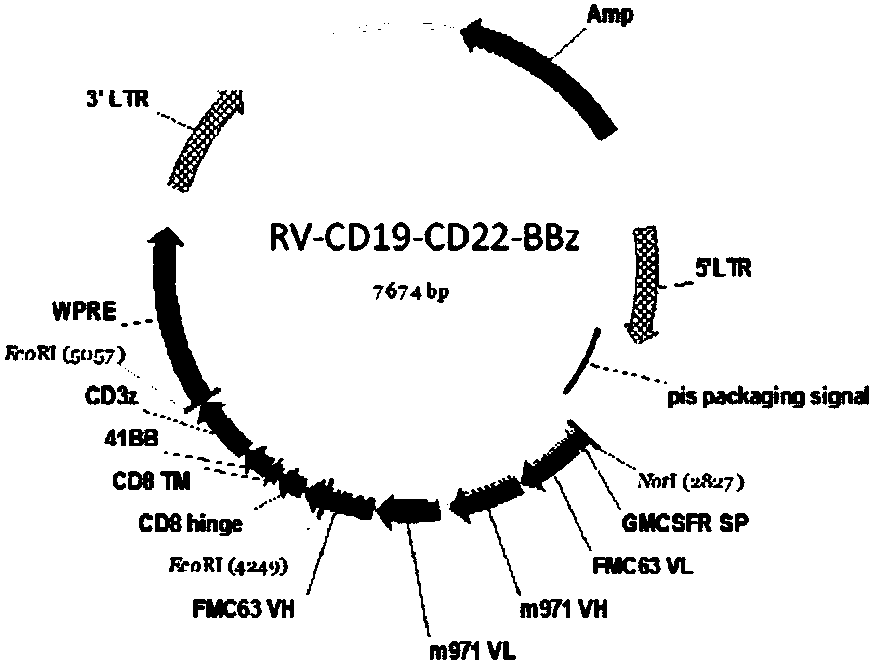
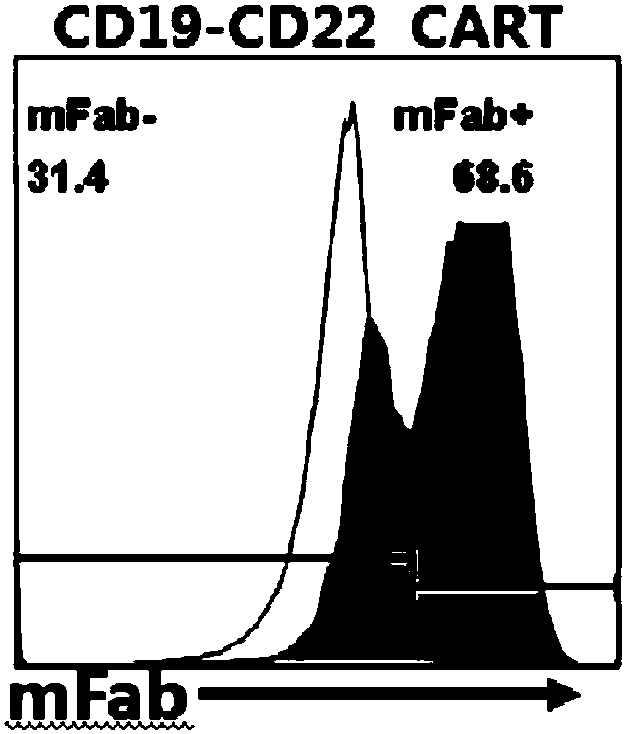
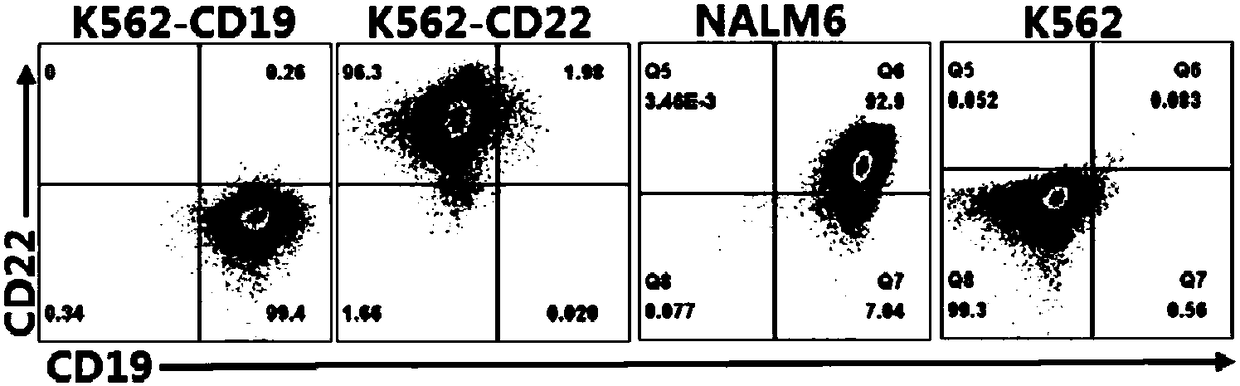
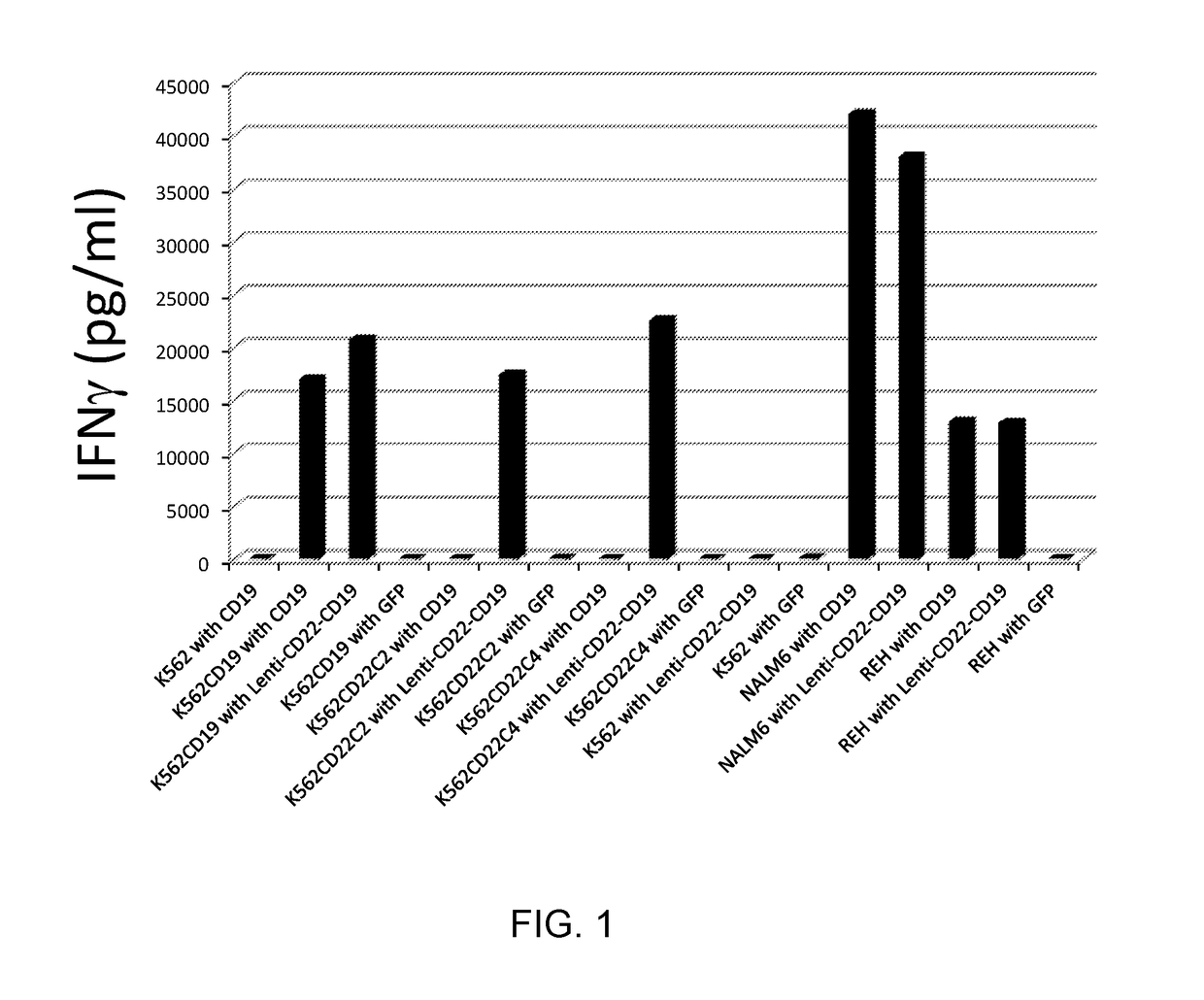
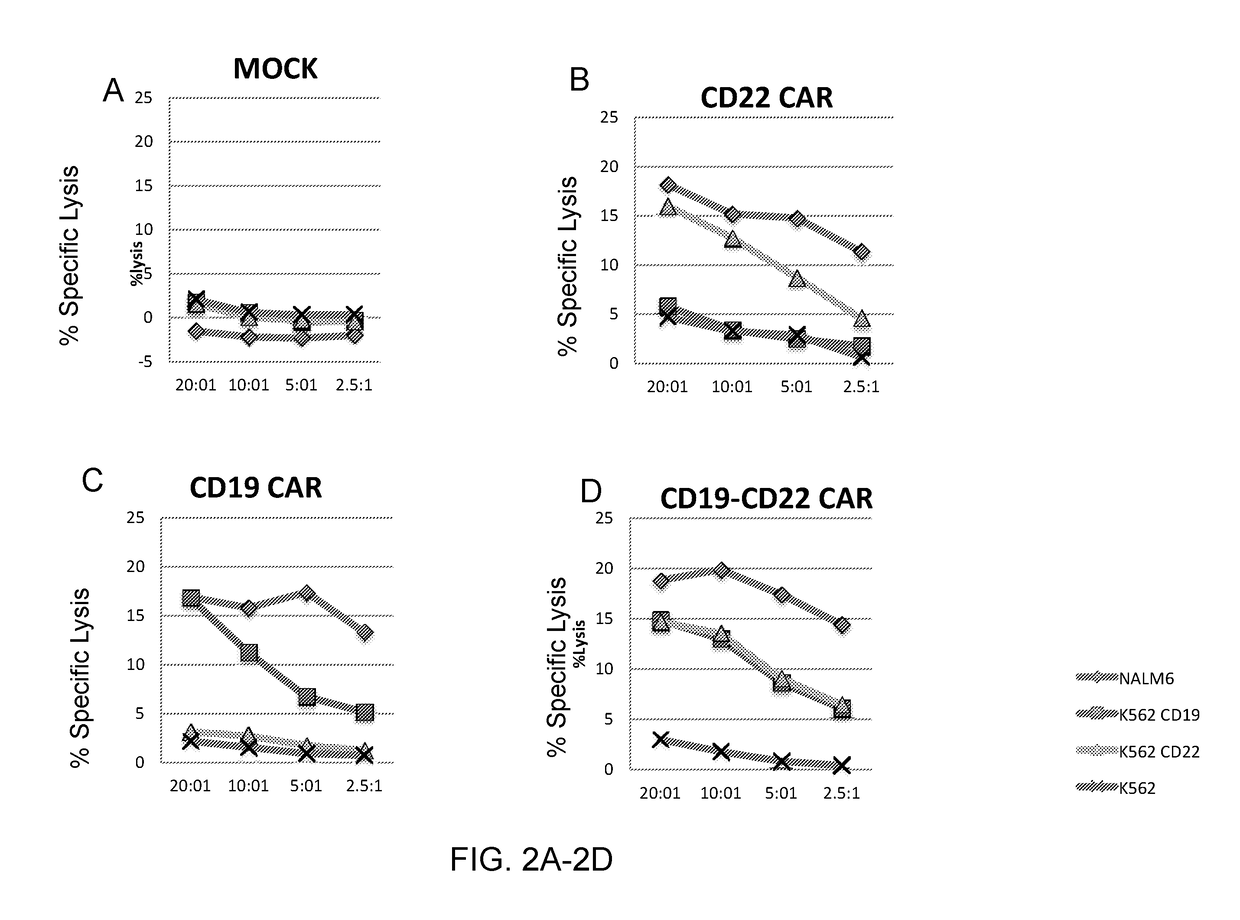
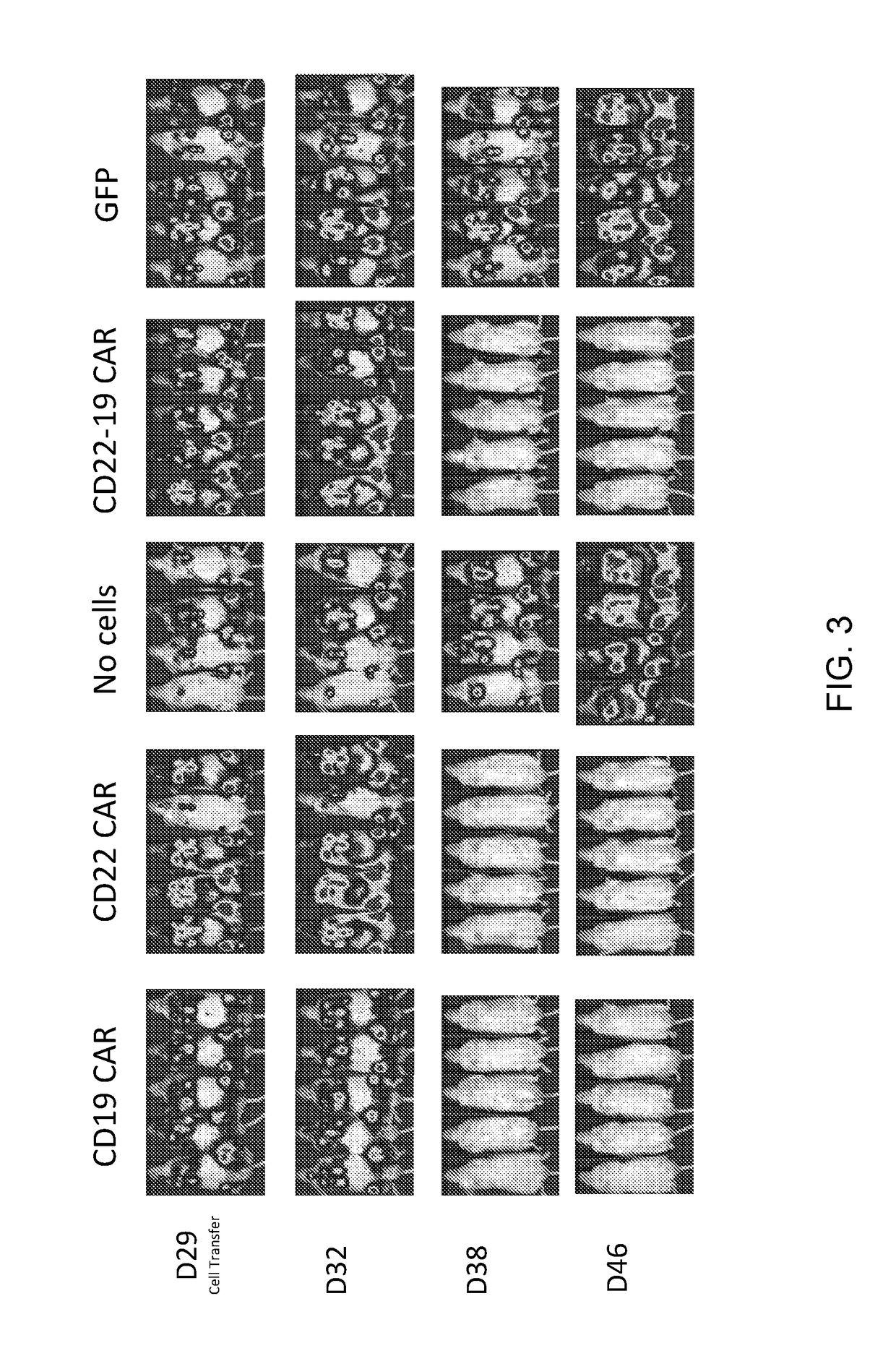
The invention relates to a double-target CD19 and CD22 chimeric antigen receptor and application thereof, in particular to a polynucleotide sequence which is selected from (1) a coding sequence comprising anti-CD22 and anti-CD19 single-chain antibodies which are connected sequentially, a coding sequence of a human CD8 hinge region, a coding sequence of human CD8 transmembrane region, a coding sequence of a human 41BB intracellular region, a coding sequence of a human CD3 zeta intracellular region and a polynucleotide sequence of a coding sequence of a fragment, comprising an extracellular domain III and a extracellular domain IV, of an optional EGFR; and (2) a complementary sequence of the (1) polynucleotide sequence. The invention further provides a relevant fusion protein, a carrier comprising the coding sequence and application of the fusion protein, the coding sequence and the carrier. The prepared CD19-CD22-BBzCAR-T cell has a strong killing function to the specific tumor cell, CD107a expression and IFN gamma secretion are high, and the killing efficiency to the target cell can reach about 80% under the situation that the effector-target ratio is 10:1.
Owner:HRAIN BIOTECHNOLOGY CO LTD
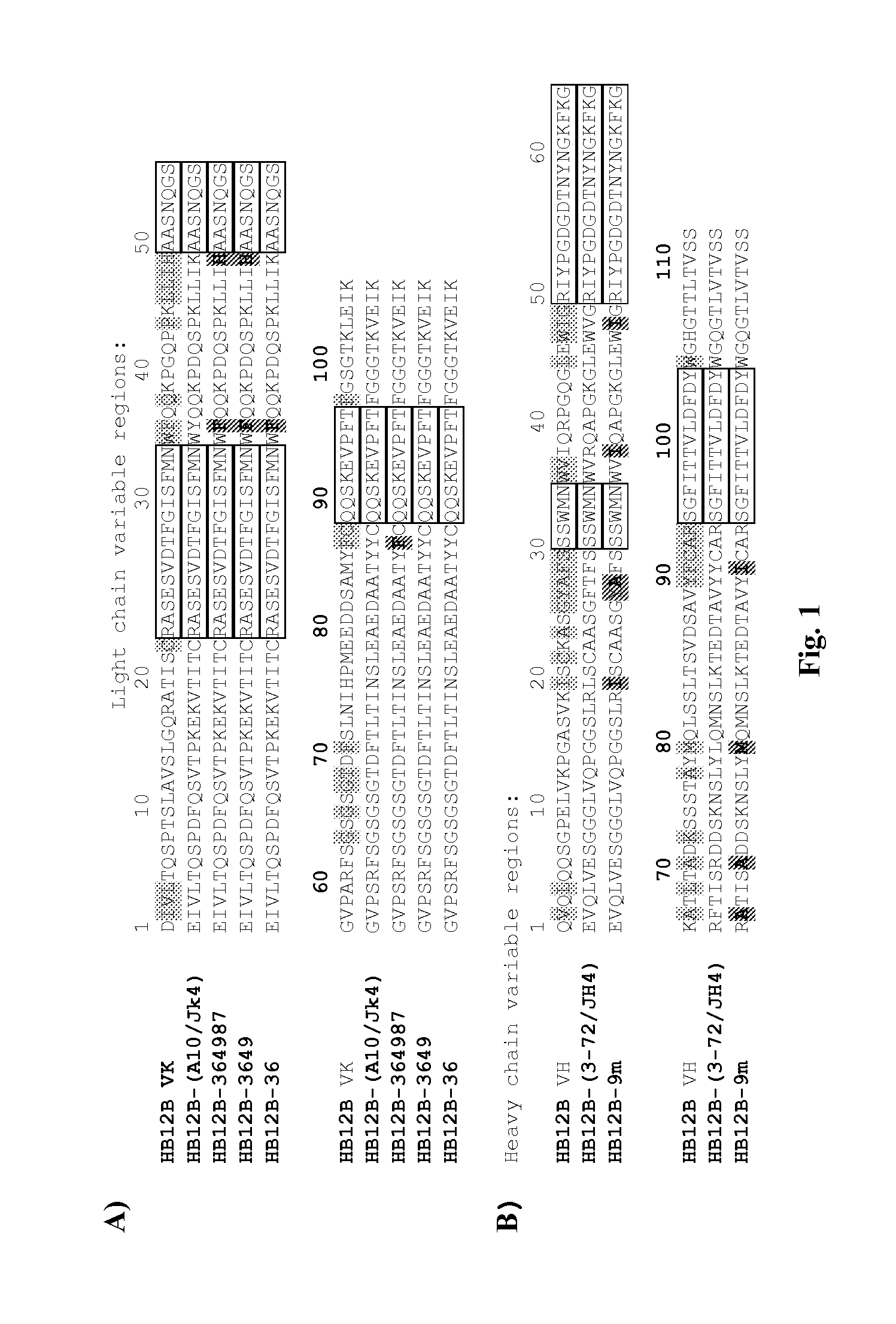
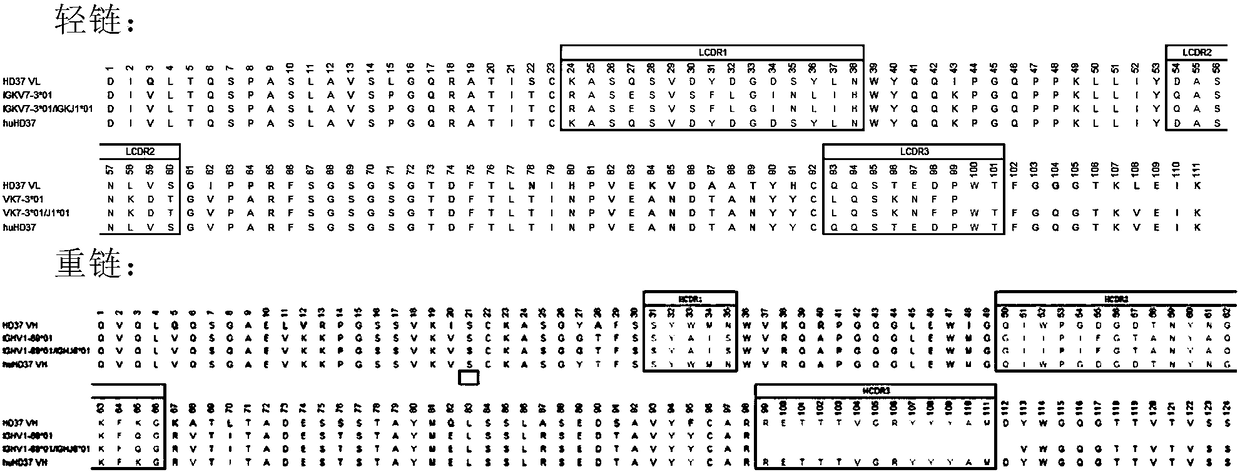
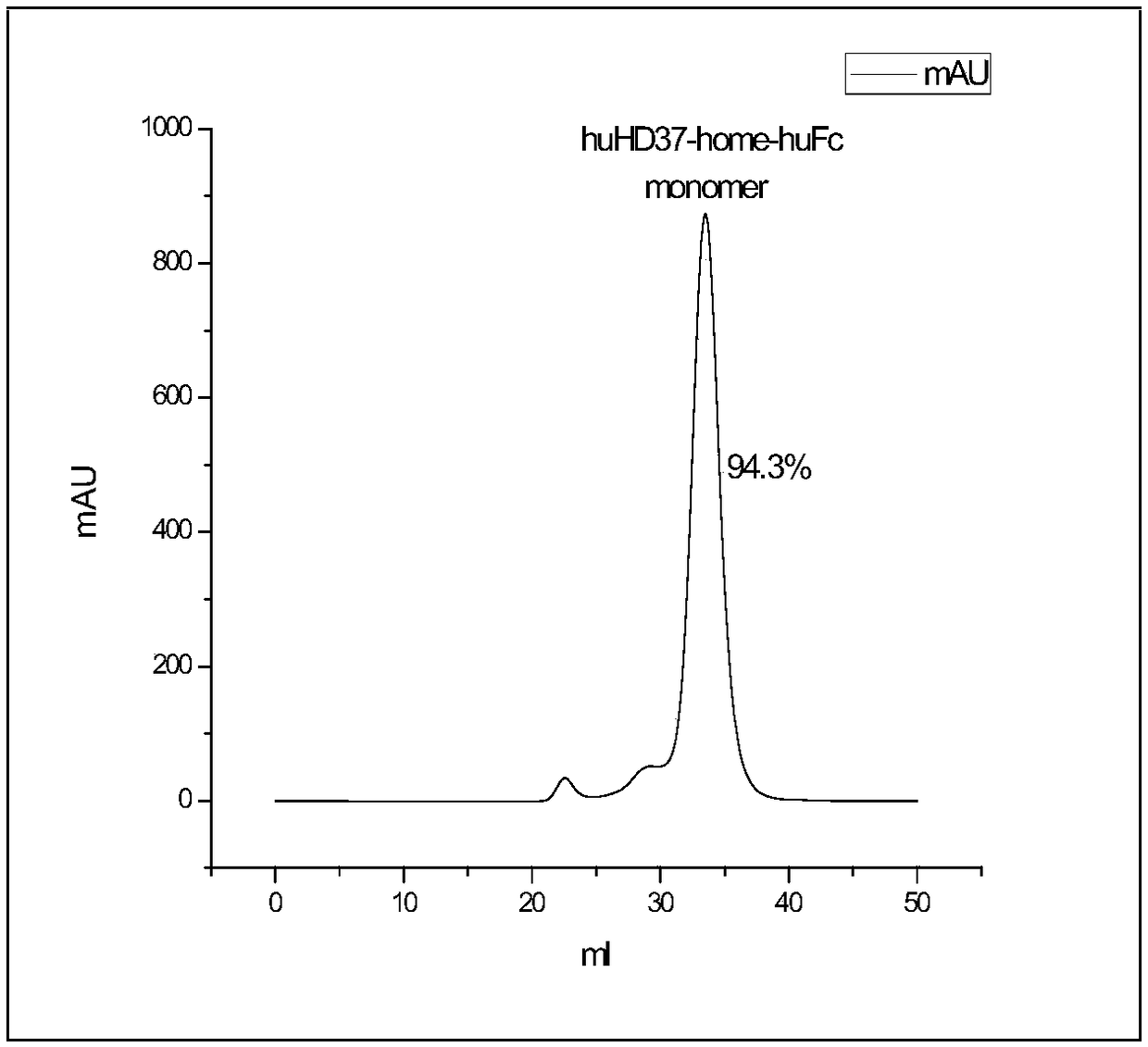
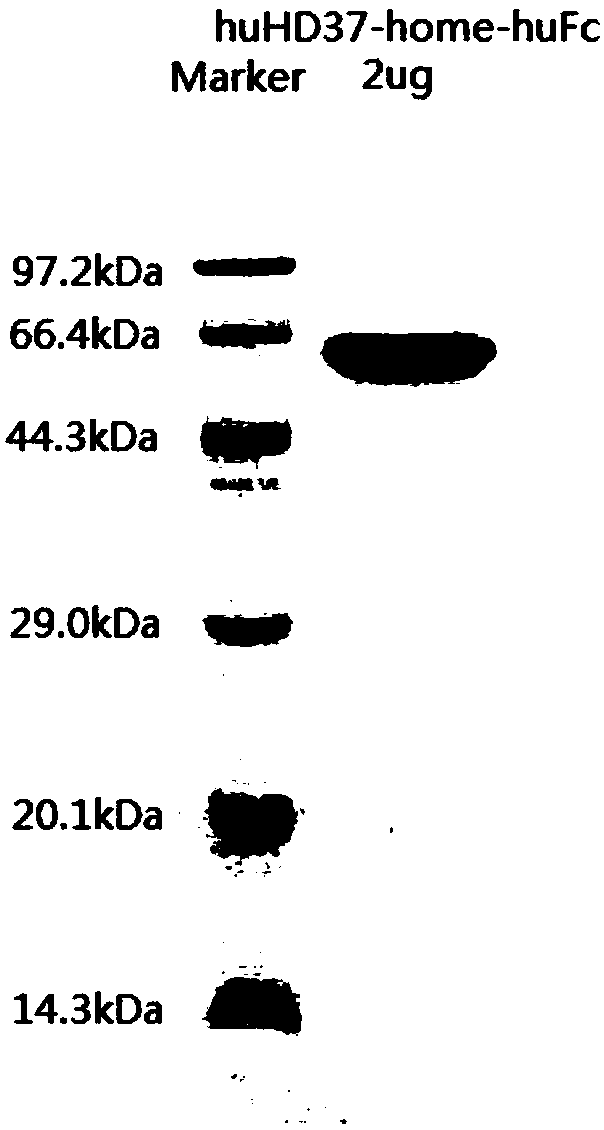
Anti-CD19 full humanized antibody or antibody segment as well as method and application thereof
ActiveCN107880128ATo achieve the purpose of healingPolypeptide with localisation/targeting motifImmunoglobulin superfamilyComplementarity determining regionHeavy chain
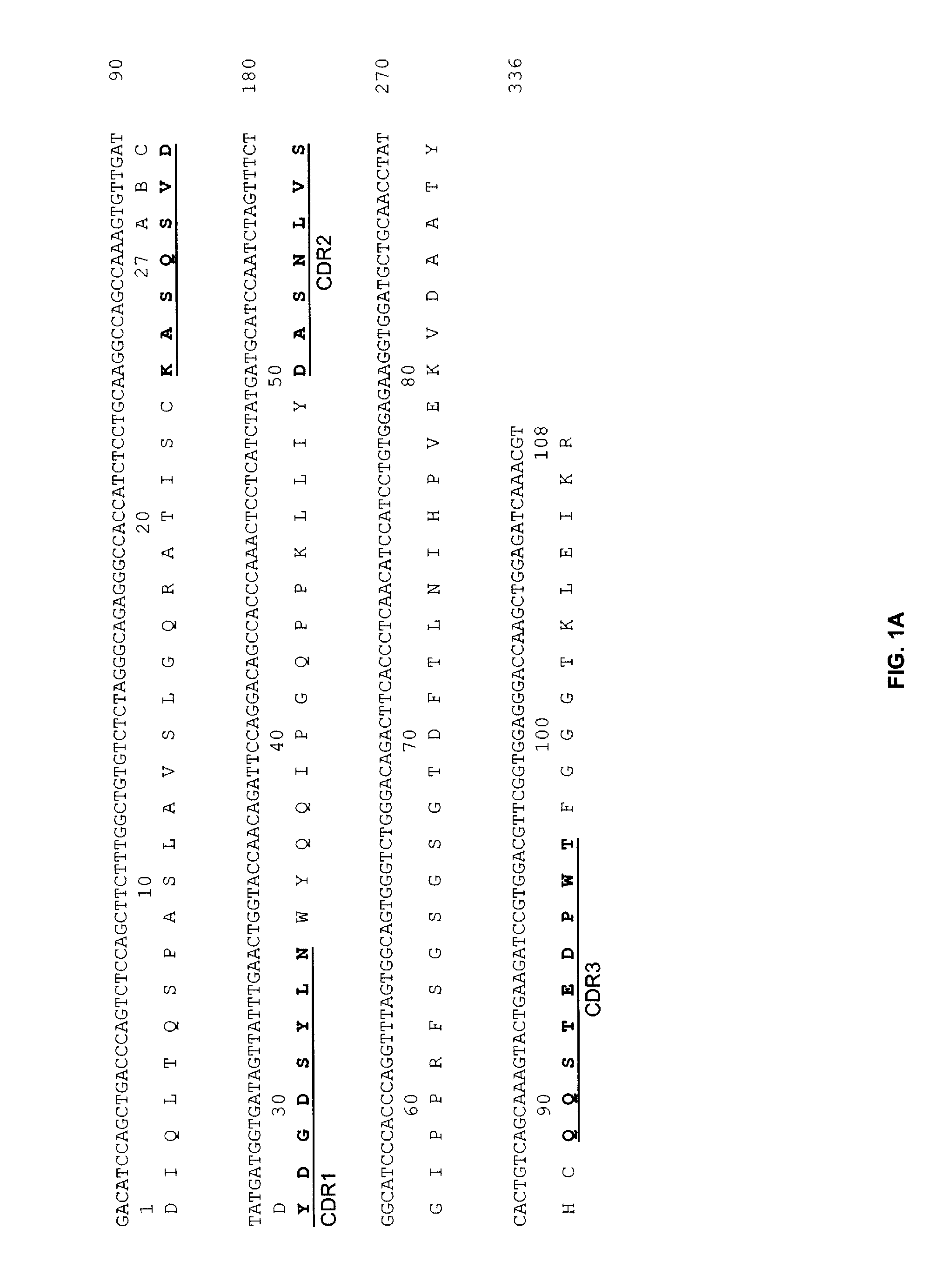
The invention discloses an anti-CD19 full humanized antibody or antibody segment as well as a method and an application thereof. The antibody or the antibody segment contains a heavy chain and a lightchain, wherein the heavy chain and the light chain comprise variable regions; the variable regions comprise complementary determining regions; the complementary determining regions CDR1, CDR2 and CDR3 of the heavy chain are separately represented by HCDR1, HCDR2 and HCDR3; the complementary determining regions CDR1, CDR2 and CDR3 of the light chain are separately represented by LCDR1, LCDR2 and LCDR3; the amino acid sequence of the HCDR1 comprises SED ID NO: 3; the amino acid sequence of the HCDR2 comprises SEQ ID NO: 4; the amino acid sequence of the HCDR3 comprises SEQ ID NO: 5; the amino acid sequence of the LCDR1 comprises SEQ ID NO: 6; and the amino acid sequence of the LCDR2 comprises SEQ ID NO: 7. The antibody or the antibody segment can be combined with a humanized CD19 protein with high specificity, and by means of engineered expression integration by means of a chimeric antigen receptor cell technology in a cell T, the obtained chimeric antigen receptor cell T can be used for treating hematological cancers related to expression of CD19.
Owner:CHANGZHOU VELOX PHARMA SCI & TECH CO LTD
Anti-PD1 and anti-CD19 bispecific antibody and applications thereof
ActiveCN106939050AImprove biological activityGood biological stabilityHybrid immunoglobulinsAntibody ingredientsCell Surface AntigensTherapeutic effect
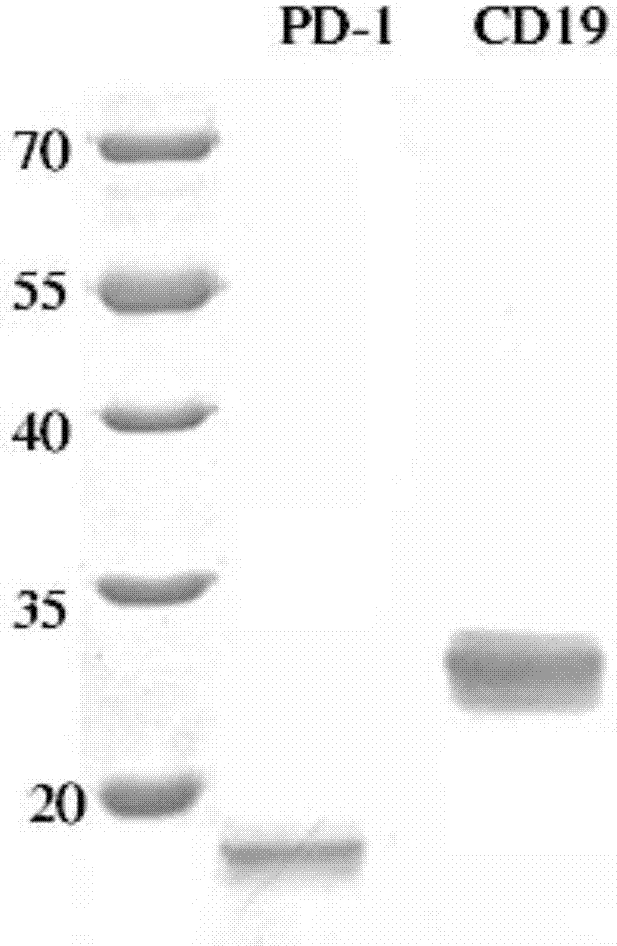
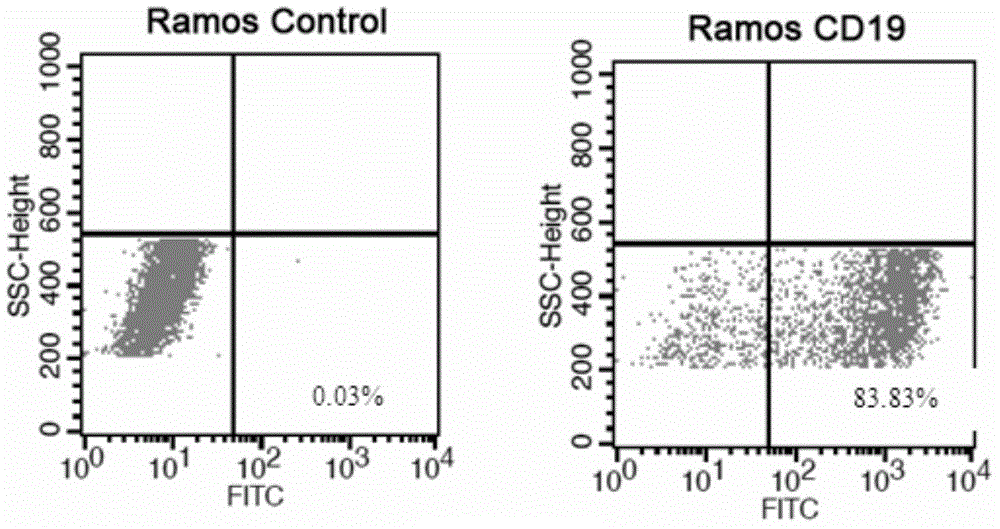
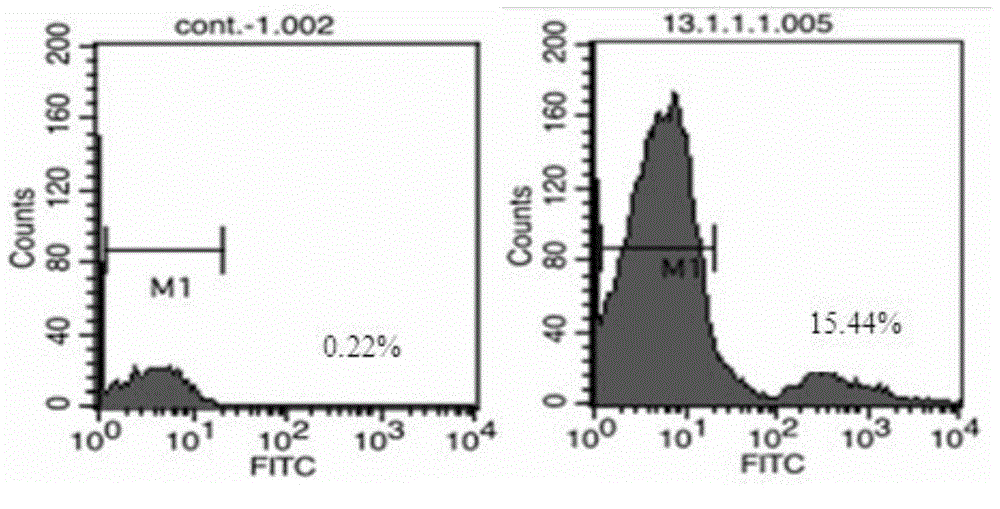
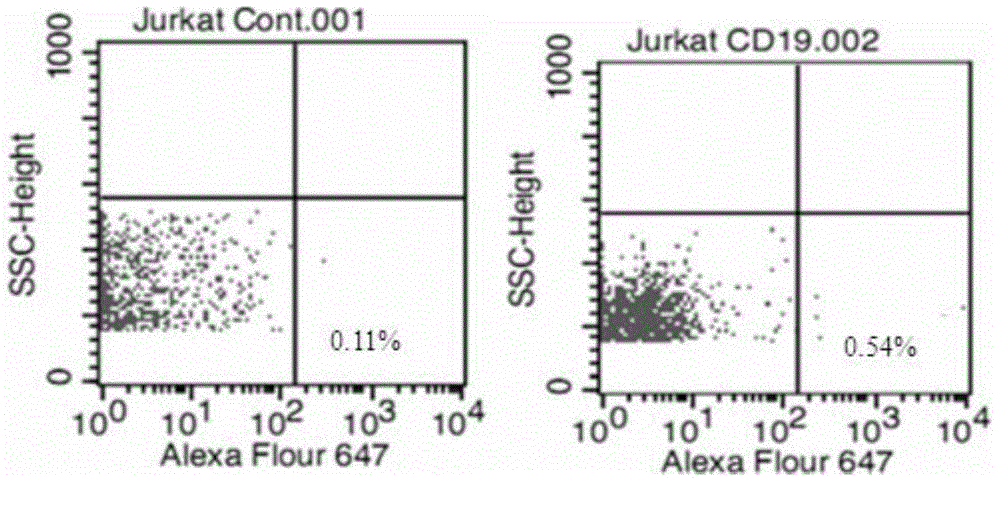
The present invention relates to anti-PD1 and anti-CD19 bispecific antibody and applications thereof, wherein the anti-PD1 and anti-CD19 bispecific antibody, or a variant, or a functional fragment thereof comprises: a domain capable of specifically recognizing and binding an immune cell surface antigen PD-1, wherein the domain comprises the heavy chain variable region (anti-PD-1 VH) of an anti-PD-1 specific antibody; and a domain capable of specifically recognizing and binding CD19, wherein the domain comprises the heavy chain variable region (anti-CD19 VH) of an anti-CD19 specific antibody. According to the present invention, the bispecific antibody has the whole human sequence, such that the occurrence of HAMA in clinical treatment application is effectively avoided, the occurrence of drug resistance can be effectively reduced, and the utilization efficiency and the treatment effect of the medicine can be improved; and the anti-PD-1 and anti-CD19 bispecific antibody can well mediate T cells to efficiently and specifically kill B-lymphocyte derived tumor cells compared to the existing anti-PD-1 specific antibody and the existing anti-CD19 specific antibody.
Owner:SHUNHAO CELL BIOTECHNOLOGY (TIANJIN) CO LTD
Anti-CD19 monoclonal antibody and preparation method thereof
ActiveCN105837689AEfficient identificationHigh affinityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsProtein targetWestern blot
The invention provides an anti-CD19 monoclonal antibody, and a preparation method and an application thereof. The anti-CD19 monoclonal antibody can effectively recognize cells with CD19 highly expressed, and can be successfully used for Western Blot detection. An affinity test shows that the monoclonal antibody has the extremely high affinity to target protein.
Owner:PERSONGEN ANKE CELLULAR THERAPEUTICS CO LTD
Treatment of cancer using humanized anti-CD19 chimeric antigen receptor
ActiveUS10221245B2Good curative effectPolypeptide with localisation/targeting motifImmunoglobulin superfamilyCD19-specific chimeric antigen receptorAntigen receptors
The invention provides compositions and methods for treating diseases associated with expression of CD19. The invention also relates to chimeric antigen receptor (CAR) specific to CD19, vectors encoding the same, and recombinant T cells comprising the CD19 CAR. The invention also includes methods of administering a genetically modified T cell expressing a CAR that comprises a CD19 binding domain.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Humanized anti-CD19 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
ActiveUS8323653B2Alleviation and mitigation and decreaseSenses disorderNervous disorderAntigenApoptosis
Owner:VIELA BIO INC
Humanized anti-CD19 antigen binding fragment
ActiveCN107383196ALow immunogenicity in vivoLong life in vivoPolypeptide with localisation/targeting motifImmunoglobulin superfamilyDiseaseSingle-Chain Antibodies
The invention provides a composition and a method for treating CD19 expression related diseases. The invention also relates to an application of humanized anti-CD19 single chain antibody with low immunogenicity as a CD19 antigen binding fragment, which is used for constructing a chimeric antigen receptor (CAR) specific to CD19, a carrier for coding the chimeric antigen receptor, and a recombined T-cell containing anti-CD19CAR. The invention also comprises a method for applying a genetically modified T-cell which expresses CAR containing the humanized anti-CD19 antigen binding fragment.
Owner:GUANGZHOU BIO GENE TECH CO LTD
Immunoregulatory antibodies and uses thereof
InactiveUS20080227198A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCD37CD23
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:BIOGEN INC
Anti-cd19 antibody therapy for autoimmune disease
The invention relates to immunotherapeutic compositions and methods for the treatment of autoimmune diseases and disorders in human subjects using therapeutic antibodies that bind to the human CD19 antigen and that preferably mediate human ADCC. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD19 antibodies of the IgG1 or IgG3 human isotype. The present invention relates to pharmaceutical compositions comprising human or humanized anti-CD19 antibodies of the IgG2 or IgG4 human isotype that preferably mediate human ADCC. The present invention also relates to pharmaceutical compositions comprising chimerized anti-CD19 antibodies of the IgG1, IgG2, IgG3, or IgG4 isotype that mediate human ADCC. In preferred embodiments, the present invention relates to pharmaceutical compositions comprising monoclonal human, humanized, or chimeric anti-CD19 antibodies.
Owner:DUKE UNIV
Immunoregulatory antibodies and uses thereof
InactiveUS20080226626A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCD37CD23
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:BIOGEN INC
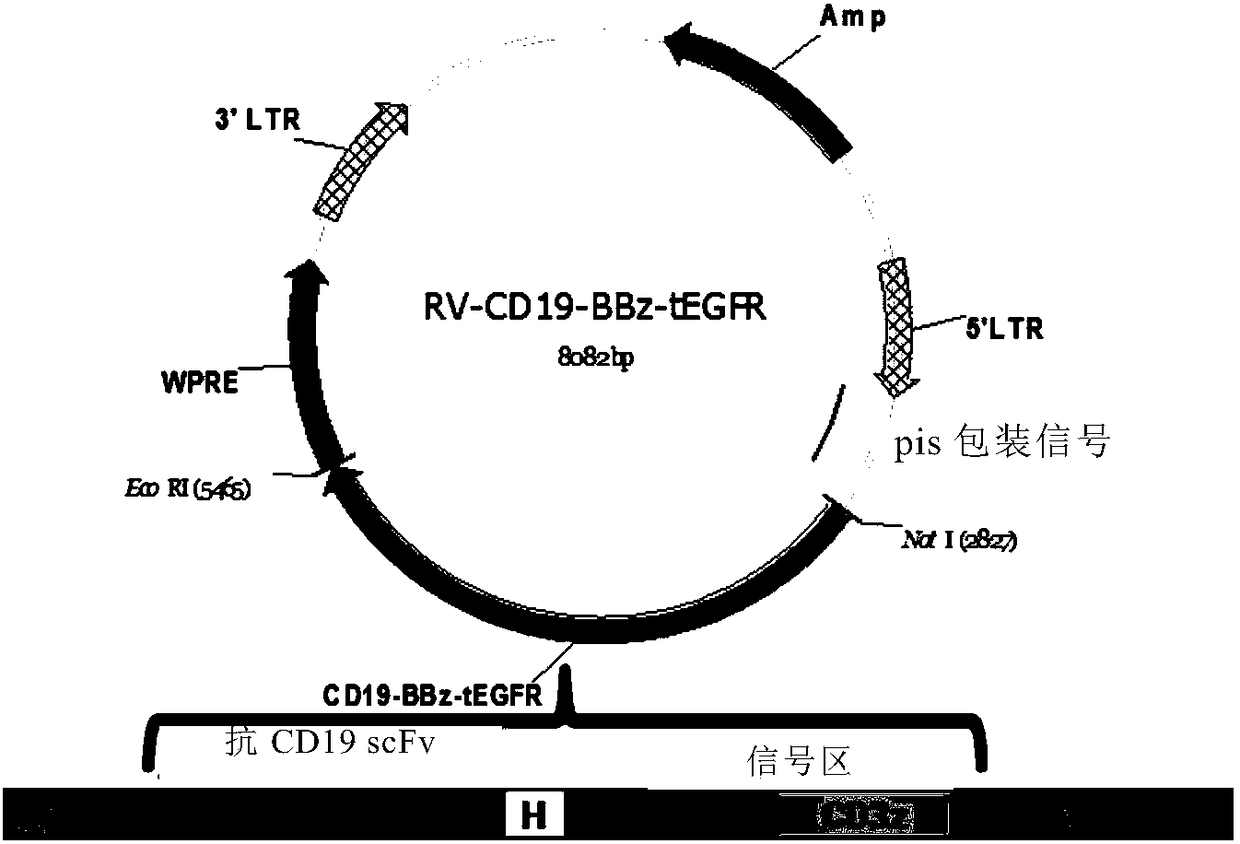
Chimeric antigen receptor targeting to CD19-41BB-tEGFR, and application thereof
ActiveCN108070607AAntibody mimetics/scaffoldsMammal material medical ingredientsSingle-Chain AntibodiesHinge region
The invention relates to a chimeric antigen receptor targeting to CD19-41BB-tEGFR, and application thereof. Specifically, the invention provides a polynucleotide sequence. The polynucleotide sequenceis selected from (1) a polynucleotide sequence containing, successively connected, a coding sequence of an anti-CD19 single-chain antibody, a coding sequence of the hinge region of human CD8alpha, a coding sequence of the transmembrane region of human CD8, a coding sequence of the intracellular region of human 41BB, a coding sequence of the hinge region of human CD8zeta and an optional coding sequence of an EGFR fragment containing an extracellular domain III and an extracellular domain IV; and (2) complementary sequences of the polynucleotide sequence described in (1). The invention also provides related fusion proteins, vectors containing the coding sequences, and application of the fusion proteins, the coding sequences and the vectors.
Owner:上海恒润达生生物制药有限公司
Humanized Anti-cd 19 antibody formulations
InactiveUS20120148576A1Enhanced effector functionImprove stabilityPharmaceutical delivery mechanismAntibody ingredientsDiseaseApoptosis
The present invention provides stable liquid formulations comprising chimeric and humanized versions of anti-CD 19 mouse monoclonal antibodies that may mediate ADCC, CDC, and / or apoptosis for the treatment of B cell diseases and disorders.
Owner:MEDIMMUNE LLC
Dual specific Anti-cd22-Anti-cd19 chimeric antigen receptors
ActiveUS20180111992A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorsAnti cd19
The invention provides dual specific chimeric antigen receptors (CARs) having antigenic specificity for CD19 and CD22. Nucleic acids, recombinant expression vectors, host cells, populations of cells, and pharmaceutical compositions relating to the CARs are disclosed. Methods of detecting the presence of cancer in a mammal and methods of treating or preventing cancer in a mammal are also disclosed.
Owner:UNITED STATES OF AMERICA
Specific antibody taking CD19 as target point, CAR-NK cell as well as preparation and application thereof
ActiveCN108047332AStable traitsSpecific killImmunoglobulin superfamilyImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseHeavy chain
The invention provides an anti-CD19 antibody taking CD19 as a target point or an antigen binding fragment, wherein the antibody contains a heavy chain variable area (VH) and / or a light chain variablearea (VL); the heavy chain variable area contains heavy chains VHCDR1, VHCDR2 and VHCDR3; and the light chain variable area contains light chains VLCDR1, VLCDR2 and VLCDR3. The anti-CD19 antibody taking CD19 as the target point or the antigen binding fragment, which is provided by the invention, an anti-CD19 CAR-NK cell structured thereby is obtained through monoclonal cell cultivation and amplification, has stable characteristic and can be applied to large-scale production and preparation. The anti-CD19 CAR-NK cell takes a CD19 molecule as a target antigen, can specifically kill or damage lymphoma cells, can serve as a medicine for treating lymphoma diseases and is applied to treatment on tumor highly expressed by the CD19 molecules.
Owner:ASCLEPIUS SUZHOU TECH CO GRP CO LTD
Anti-CD19 antibodies and uses in oncology
The present invention relates to compositions and methods for the treatment of B-cell diseases and disorders in a subject using therapeutic antibodies that bind to the human CD19 antigen, preferably mediate human antibody-dependent cell-mediated cytotoxicity (ADCC), Said disease + such as, but not limited to, B-cell tumors. The present invention relates to pharmaceutical compositions comprising anti-CD19 antibodies of human or humanized IgG1 and / or IgG3 human isotype. The present invention relates to pharmaceutical compositions comprising human, humanized or chimeric IgG2 and / or IgG4 human isotypes, preferably anti-CD19 antibodies that mediate ADCC in humans. The invention also relates to chimeric anti-CD19 antibodies comprising an IgGl, IgG2, IgG3 or IgG4 isotype that mediates ADCC in humans. In a preferred embodiment, the present invention relates to a pharmaceutical composition comprising a monoclonal human, humanized or chimeric anti-CD19 antibody.
Owner:DUKE UNIV
Anti-CD3/anti-CD19 dual-specific antibody and application thereof
InactiveCN105017422AHigh affinityAvoid side effectsImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsSingle-Chain AntibodiesSide effect
The invention discloses an anti-CD3 / anti-CD19 dual-specific antibody and an application thereof. The anti-CD3 / anti-CD19 dual-specific antibody comprises the combined structural domain of a completely-humanized anti-CD3 single-chain antibody and a completely-humanized anti-CD19 single-chain antibody. The variable region amino acid sequence of heavy chains and light chains of the completely-humanized anti-CD3 single-chain antibody is shown as the SEQ ID NO:7-8. The variable region amino acid sequence of heavy chains and light chains of the completely-humanized anti-CD19 single-chain antibody is shown as the SEQ ID NO:15-16. The anti-CD3 / anti-CD19 dual-specific antibody has high affinity to CD3 molecules and CD19 molecules, and has the effect of conducting mediated activation on T lymphocytes to kill tumor cells. Moreover, the heavy chains and the light chains of the antibody molecules are completely humanized, and thus plenty of side effects are avoided.
Owner:SHANDONG BAYONN PHARMA TECH
Humanized antibody against CD19 and immune effector cells targeting to CD19
The invention relates to an anti-CD19 humanized antibody prepared from a murine monoclonal antibody, a chimeric antigen receptor containing the humanized antibody, and immune cells expressing the humanized antibody. The humanized antibody of the invention does not produce an anti-antibody reaction (AAR) and a human anti-mouse antibody reaction (HAMA), has better affinity than a murine antibody, and presents excellent activity and safety, so a novel means is provided for treating tumors expressing CD19.
Owner:CARSGEN THERAPEUTICS
Anti CD19 engineered antibody for target conjugated lymphocyte, leuco cyte and its use
InactiveCN1775808AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsTriturationGenetic engineering
The invention discloses an anti-CD19 engineering antibody and its application which is used in target direction combining lymphocytic leukemia cell. It lays a foundation for the next trituration genetic engineering medicine of the target direction therapy leukemia. It relates to anti-CD19 monoclonal antibody HI19a heavy and light chain variable region gene, and application of the gene code polypeptide, the gene carrier, and using the gene and polypeptide to make leukemia therapy medicine. The heavy and light chain variable region gene is come from the anti-CD19 monoclonal antibody HI19a. The invention successfully adopts gene engineering technique to make anti-CD19 gene engineering antibody, and lays a foundation for the target direction therapy of the leukemia.
Owner:INST OF HEMATOLOGY & BLOOD HOSPITAL CHINESE ACAD OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com