Pharmaceutical compositions comprising bispecific anti-cd3, anti-cd19 antibody constructs for the treatment of b-cell related disorders
A bispecific, combination technology, applied in the direction of drug combination, antibody, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve the problems of promoting allergic and inflammatory events
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1: CD19xCD3 and CD3xCD19 Single Chain Bispecifics Comprising Different Domain Rearrangements
[0083] Antibody construction
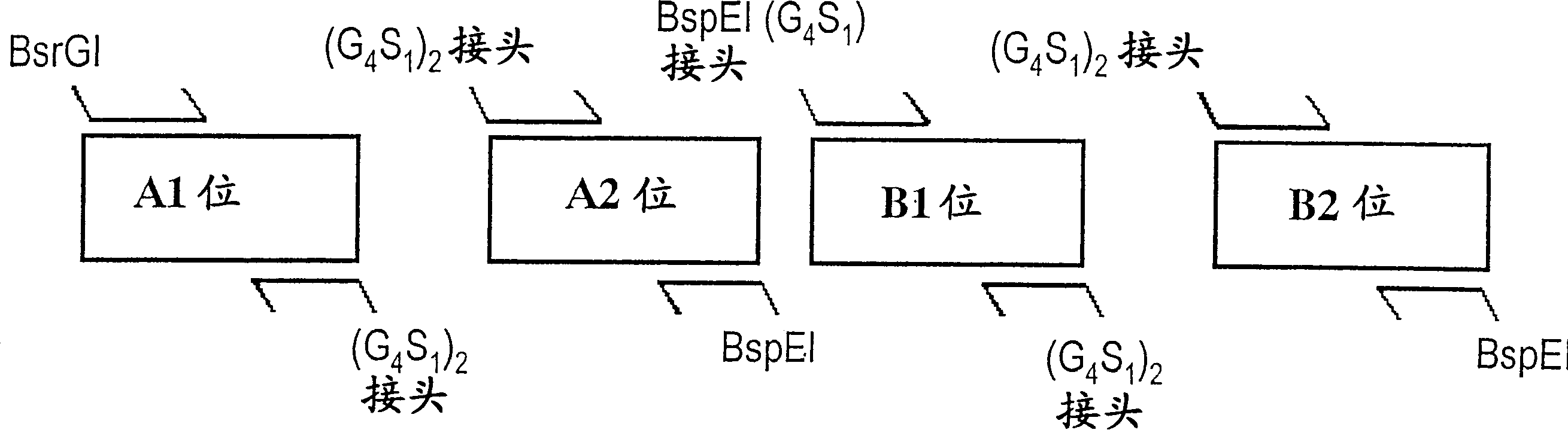
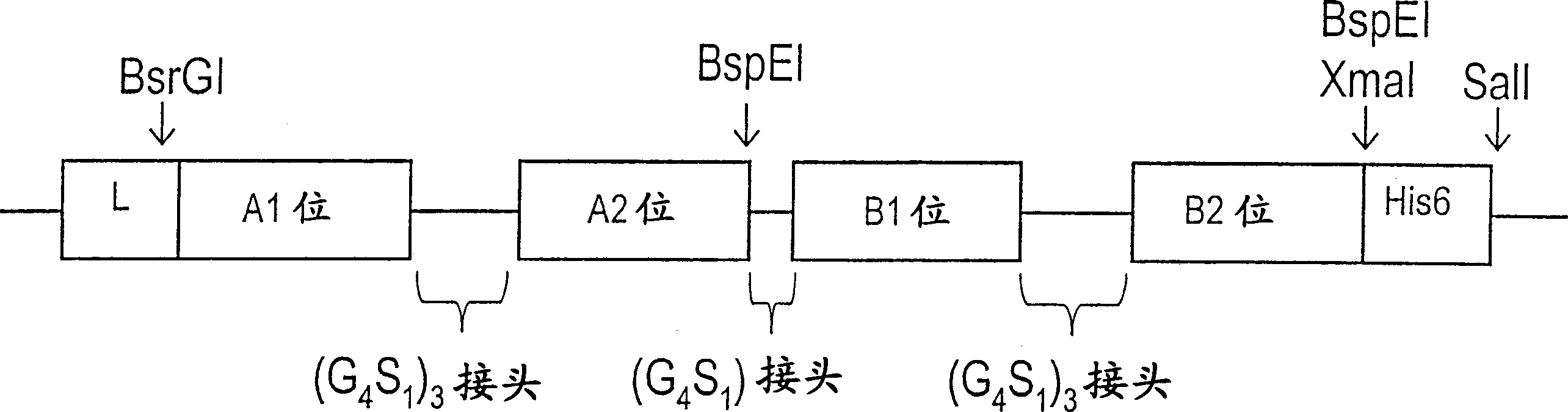
[0084] Typically, bispecific single chain antibody molecules comprising human CD3 antigen binding specificity domains and human CD19 antigen binding specificity domains are designed as shown in Table 1 below:
[0085] Constructed
No
SEQ ID Nos
(nuc / prot)
protein construct form
(N→C)
1
29 / 30
VL(CD19)-VH(CD19)-VH(CD3)-VL(CD3)
2
1 / 2
VH(CD19)-VL(CD19)-VH(CD3)-VL(CD3)
3
3 / 4
VL(CD19)-VH(CD19)-VL(CD3)-VH(CD3)
4
5 / 6
VH(CD19)-VL(CD19)-VL(CD3)-VH(CD3)
5
7 / 8
VL(CD3)-VH(CD3)-VH(CD19)-VL(CD19)
6
9 / 10
VH(CD3)-VL(CD3)-VH(CD19)-VL(CD19)
7
11 / 12
VL(CD3)-VH(CD3)-VL(CD19)-VH(CD19)
8
13 / 14
VH(CD3)-VL(CD3)-VL(CD19)-VH(CD19)
[0086]Variable light (VL) and variable heavy ...
Embodiment 2
[0097] Example 2: Expression and purification of single chain bispecific antibodies against CD3 and CD19
[0098] The protein was expressed in Chinese hamster egg cells (CHO). Expression vectors were transfected after treatment of cells with calcium phosphate ("Molecular Cloning", Sambrook et. al. 1989). Cells were cultured in CHO-modified DMEM medium in spinner flasks for 7 days prior to harvest. Cells were removed by centrifugation and the supernatant containing the expressed protein was stored at -20°C.
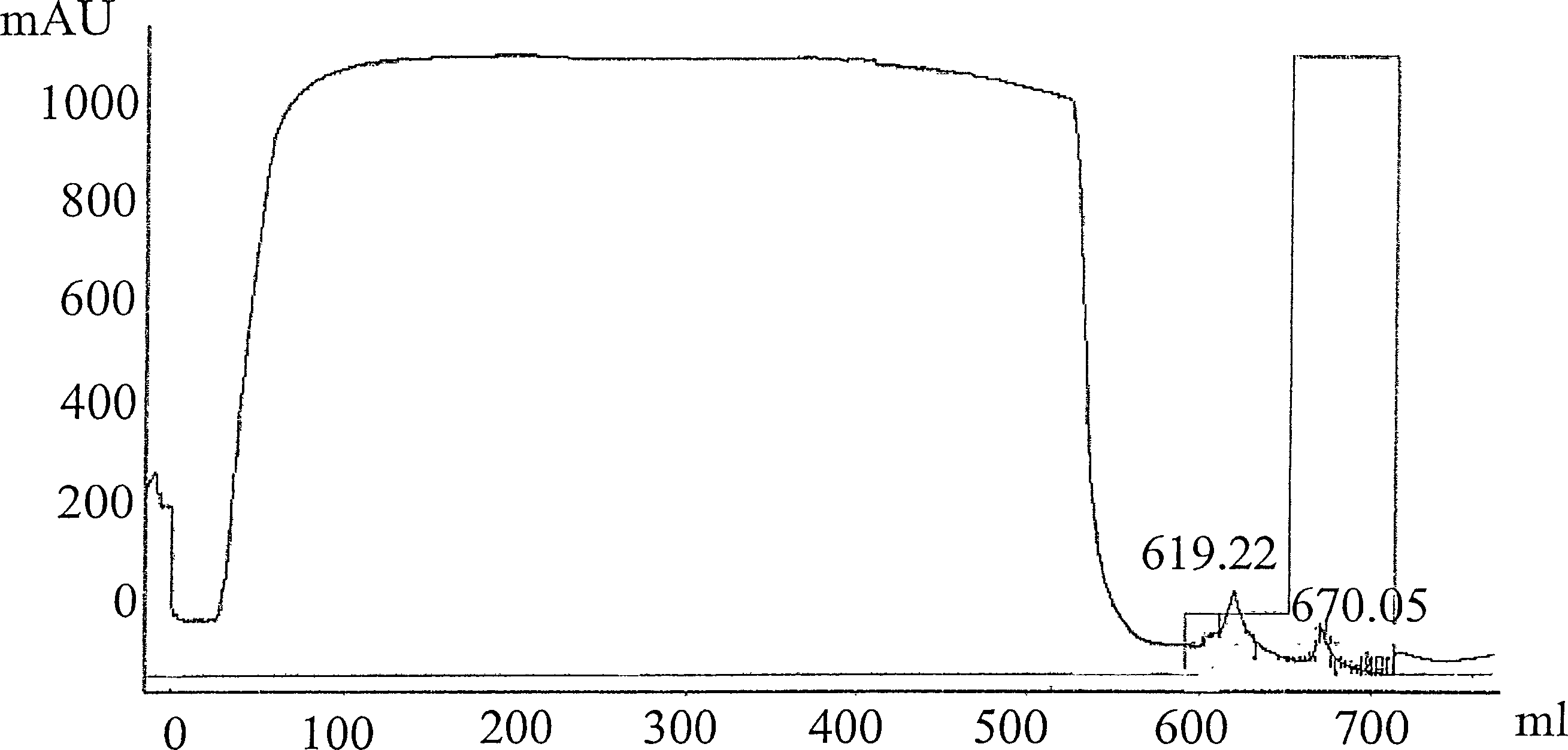
[0099] kta FPLC system (Pharmacia) and Unicorn Software is used for chromatography. All chemicals were of research grade and were purchased from Sigma (Deisenhofen) or Merck (Darmstadt). Load ZnCl with the manufacturer's protocol 2 Fractogel The column (Merck) was subjected to solid phase metal affinity chromatography ("IMAC"). The column was equilibrated with A2 buffer (20 mM sodium phosphate buffer, pH 7.5, 0.4 M NaCl), and the cell culture supernatant (50...
Embodiment 3
[0107] Example 3: Flow cytometric binding analysis of CD19xCD3-specific polypeptides
[0108] To test the function of the constructs with respect to CD19 and CD3 binding capacity, flow cytometry analysis (FACS) was performed. For this purpose, CD19-positive Nalm 6 cells (human B-cell precursor leukemia) and CD3-positive Jurkat cells (human T-cell leukemia) were used. 200,000 Nalm 6 cells and 200,000 Jurkat cells were each incubated on ice for 30 min with 50 μl of pure cell supernatant from a culture of CHO cells expressing bispecifics with different arrangements of VH and VL domains for CD19 and CD3, respectively Antibodies (as described in Example 2). Cells were washed twice with PBS and binding of the constructs was detected as follows. Cells treated as described above were contacted with an unlabeled murine penta-histidine antibody (diluted 1:20 in 50 μl PBS with 2% FCS; Qiagen; Order No. 34660), which passed the C of the construct The terminal histidine tag specifically...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com