Humanized anti-CD19 antigen binding fragment
A technology of combining fragments and humanization, applied in DNA/RNA fragments, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, medical raw materials derived from mammals, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1 Homology Modeling of Murine Antibody FMC63 scFv Chain
[0064] a. The principle of homology modeling is that similar sequences lead to similar structures, and the conservation of structures is much greater than that of sequences.
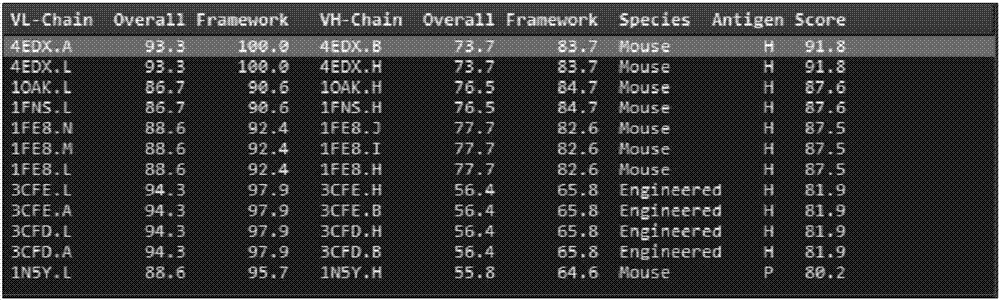
[0065] (1) First, search and compare homologous sequences in the NCBI database, and select a sequence with high homology to the amino acid sequence of the FMC63 scFv chain as a homologous template. The selected sequence should at least meet the following three conditions: ① sequence The similarity should be greater than 30%; ② High sequence identity and E value should be small enough (figure 1 shown.
[0066] The homologous template selected in the present invention is the crystal structure of the mouse monoclonal antibody Fab, and the PDB database number is: 4EDX.
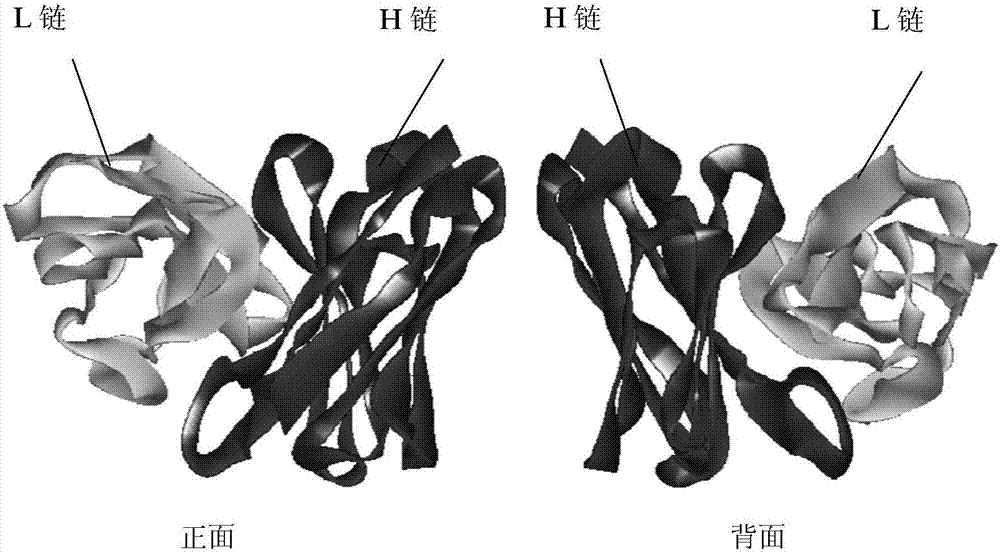
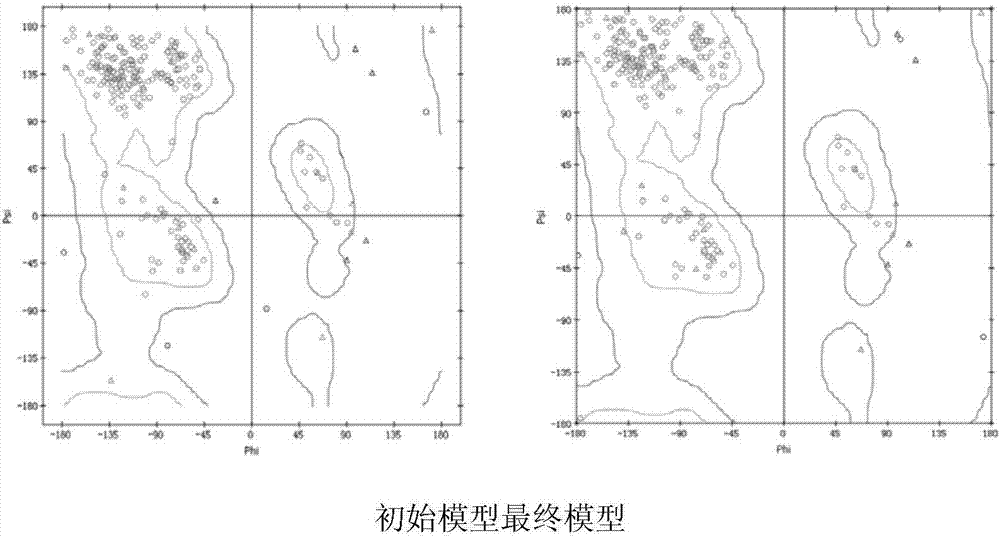
[0067] After the sequence alignment was completed, the Antibody Modeler module of the MOE software was used to perform homology modeling on the FMC63 scFv chain, and A...
Embodiment 2
[0083] Example 2. IgBlast analysis and antigen affinity prediction
[0084] a. The following is the sequence of the light chain variable region of the mouse anti-CD19 antibody FMC63, the underlined part is the mouse anti-framework sequence (FR sequence), and the bold part is the CDR region sequence.
[0085] Seq ID No: 29
[0086] DIQMTQTTSSLSASLGDRVTISCRASQDISKYLNWYQQKPDGTVKLLIYHTSRLHSGVPSRFSGSGSGTDYSLTISNLEQEDIATYFCQQGNTLPYTFGGGTKLEIT
[0087] By analyzing the light chain variable region sequence of the mouse anti-CD19 antibody FMC63, the human germline antibody gene library (NCBI IgBLAST) was compared according to its light chain variable region FR1, FR2, FR3 and FR4 and the entire light chain sequence, Find the corresponding sequence of the variable region of the human germline (germline) antibody (less immunogenic than the mature antibody) similar to the sequence of the mouse anti-CD19 light chain variable region FR1, FR2, FR3 and FR4. Figure 4 It is the FR1 sequence c...
Embodiment 3
[0206] The synthesis of embodiment 3CAR gene and the construction of carrier
[0207] Select the five humanized sequences in Example 2, and add the linker sequence (gly4ser)3 between VL and VH in turn, so the structure of the scFv part designed and constructed into the chimeric antigen receptor is: VL-(gly4ser)3- VH. Specifically, the amino acid sequences of the five ScFv obtained are shown in sequence in Seq ID.No.46-Seq ID.No.50; the corresponding nucleic acid sequences are shown in SeqID.No.51-Seq ID.No.55 . details as follows:
[0208] The amino acid sequence of the scFv corresponding to the humanized sequence one (H1) is shown in Seq ID.No.46.
[0209] The amino acid sequence of the scFv corresponding to the humanized sequence 2 (H2) is shown in Seq ID.No.47.
[0210] The amino acid sequence of the scFv corresponding to the humanized sequence three (H3) is shown in Seq ID.No.48.
[0211] The amino acid sequence of the scFv corresponding to the humanized sequence four...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com