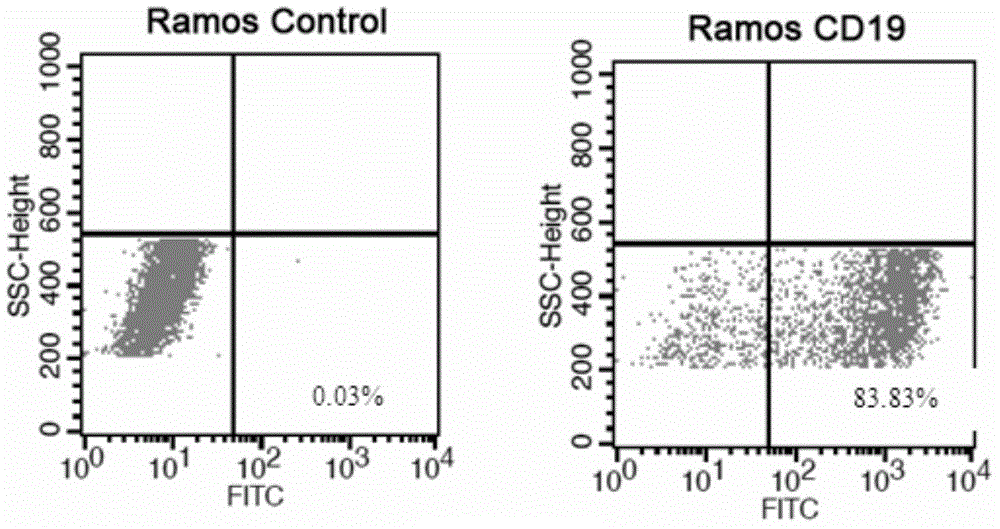
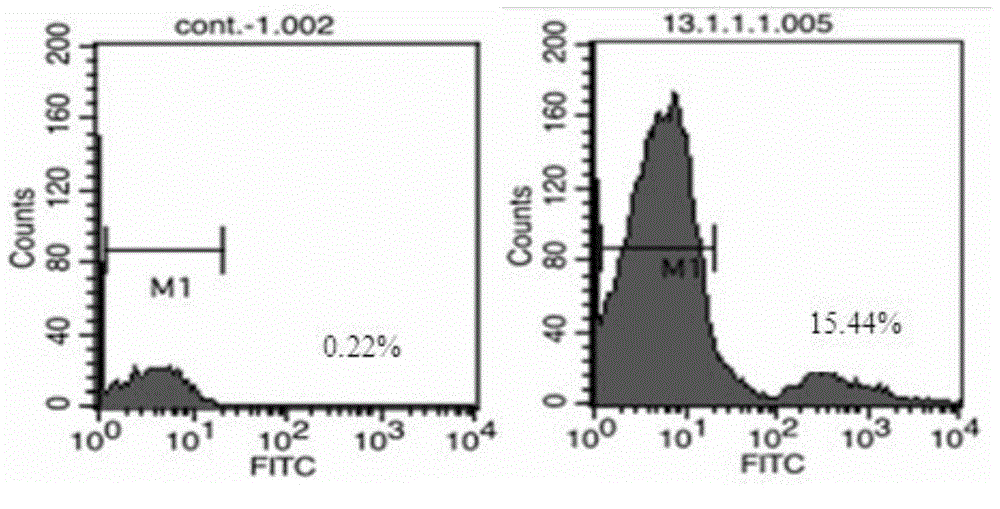
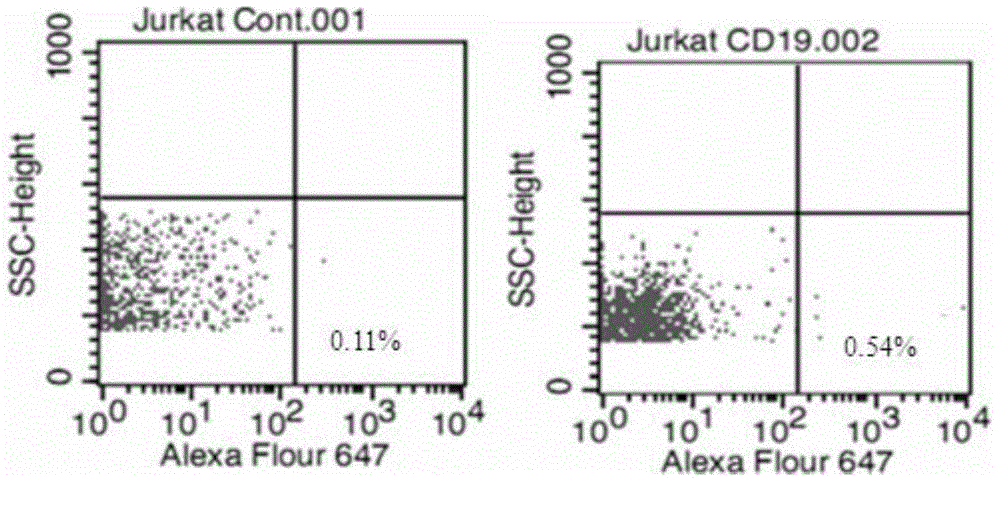
Anti-CD19 monoclonal antibody and preparation method thereof
An antibody and CDR2 technology, applied in the field of biomedicine, can solve the problems of unsatisfactory performance of CD19 protein antibody
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0151] Preparation of monoclonal antibodies
[0152] Antibodies of the present invention can be prepared by various techniques known to those skilled in the art. For example, an antigen of the invention may be administered to an animal to induce the production of monoclonal antibodies. For monoclonal antibodies, hybridoma technology can be used to prepare (see Kohler et al., Nature 256; 495, 1975; Kohler et al., Eur.J.Immunol.6:511, 1976; Kohler et al., Eur.J.Immunol. 6:292,1976; Hammerling et al., In Monoclonal Antibodies and T Cell Hybridomas, Elsevier, N.Y., 1981) or can be prepared by recombinant DNA method (US Patent No. 4,816,567).
[0153] Representative myeloma cells are those that fuse efficiently, support stable high-level production of antibody by selected antibody-producing cells, and are sensitive to culture medium (HAT medium matrix), including myeloma cell lines, such as murine Myeloma cell lines, including those derived from MOPC-21 and MPC-11 mouse tumors (a...
Embodiment 1
[0184] Step 1: Preparation of hybridoma cells
[0185]Immune female healthy BALB / c mice with B cell line tumor cells or CD19 eukaryotic recombinant protein antigen, select the mice with positive serum ELISA, WB, FC, extract spleen cells from them, and fuse with myeloma cells , forming hybridoma cells; hybridoma cells were cultured in HAT medium, and the monoclonal cells that were positive in high-throughput flow cytometry screening were re-screened by ELISA to obtain 36 positive clones, and then re-screened by WB to obtain positive clones 9 strains were subcloned to WB-positive cells. After 2 times of subcloning, 6 strains of negative cell strains during the subcloning process were excluded, and finally 3 strains of monoclonal cells capable of stably secreting CD19 antibodies were selected (including: PGC3D6H10 monoclonal antibody cell line, PGA6E2D5 monoclonal antibody cell line, PGA6D5C6 monoclonal antibody cell line); use the subtype identification kit to identify the subty...
Embodiment 2
[0199] Cloning of Example 2 Anti-CD19 Monoclonal Antibody Heavy Chain and Light Chain Variable Region Genes
[0200] The cell line used is a hybridoma cell line that can secrete high-affinity and high-specificity anti-CD19 monoclonal antibody obtained by the above method, the corresponding number is PGC3D6H10, and the corresponding antibody molecular subtypes are: heavy chain IgG1 type, light Chain kappa type.
[0201] Take hybridoma cells in logarithmic growth phase 2×10 6 , Tri zol method to extract total cellular RNA,
[0202] A small amount was quantified by Nanodrop and detected by 1% non-denaturing agarose gel electrophoresis, then the cDNA was reverse-transcribed with SuperScript.I II First-Strand Synthesis System for RT-PCR kit (K1622, Thermo), and the specific primer was used to amplify the anti- The heavy or light chain variable region of the CD19 antibody gene. The PCR reaction product containing the corresponding heavy chain variable region or light chain variab...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com