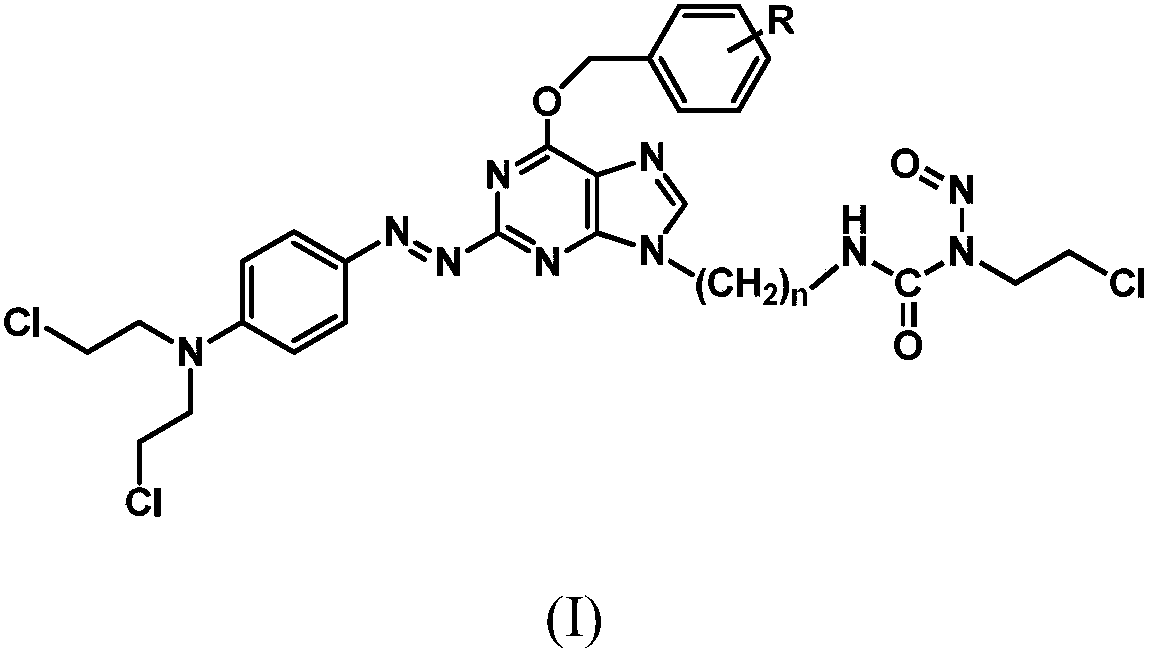
Azo aryl nitrogen mustard-chloroethylnitrosourea coupled compound, and preparation method and application thereof
A compound and composition technology, applied in the preparation of antitumor drugs, in the field of the synthesis of aromatic nitrogen mustard-chloroethyl nitrosourea coupling molecules, can solve the problem of enhanced toxicity and side effects of CENUs and no tumor targeting And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
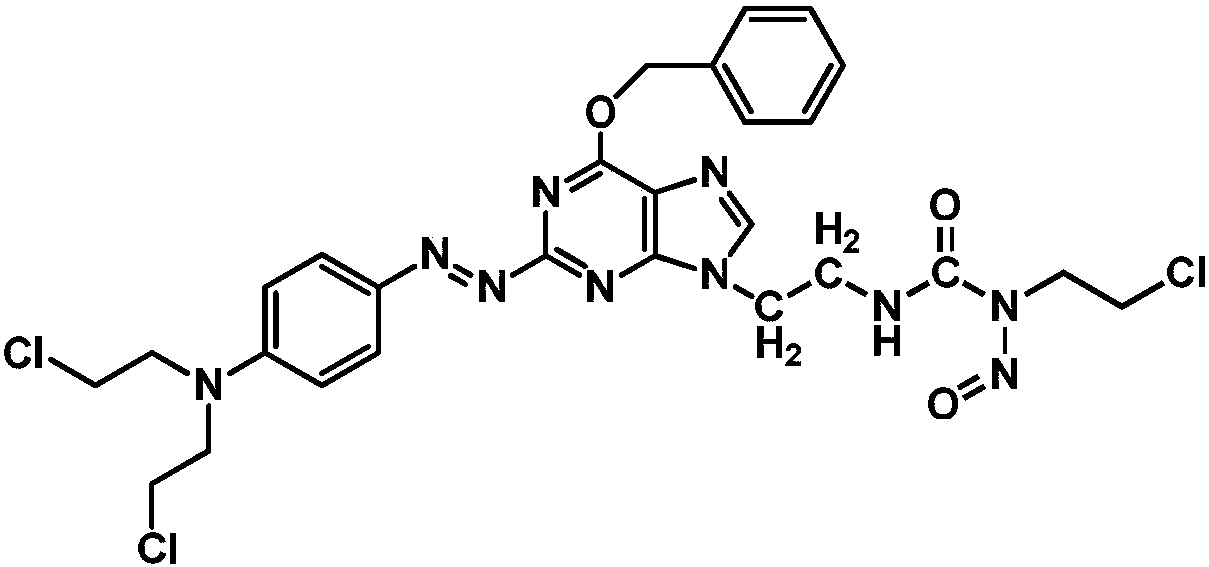
[0059] Example 1: (E)-3-(2-(6-(benzyloxy)-2-((4-(bis(2-chloroethyl)amino)phenyl)diazenyl)-9H- Synthesis of purin-9-yl)ethyl)-1-(2-chloroethyl)-1-nitrosourea (compound 1) 1) N9-bromoethyl-O 6 - Synthesis of Benzylguanine
[0060] Weigh O 6 -Benzylguanine (2.05g, 8.5mmol), anhydrous potassium carbonate (3.35g, 24.3mmol) were added to a 100mL round bottom flask, 50mL of acetone was added, the temperature was slowly raised to 52°C, and 1,2-dibromo was added dropwise Ethane (2.89 mL, 33 mmol), continued to react for 72 h after dripping, the reaction solution was filtered, the filtrate was collected, the solvent was evaporated under reduced pressure at 40°C, and then the solvent was separated and purified by silica gel column chromatography. The eluents were petroleum ether and acetic acid. Ethyl ester, gradient elution was adopted, the volume ratio of petroleum ether / ethyl acetate was gradually increased from 1:2 to 1:4, and the white solid N9-bromoethyl-O was obtained by vacuum ...
Embodiment 2
[0101] Example 2: (E)-3-(2-(6-(benzyloxy)-2-((4-(bis(2-chloroethyl)amino)phenyl)diazenyl)-9H- Synthesis of Purin-9-yl)ethyl)-1-(2-chloroethyl)-1-nitrosourea (Compound 1) 1) N9-Bromoethyl-O 6 -Synthesis of benzylguanine
[0102] Weigh O 6 - Benzylguanine (2.12g, 8.8mmol), anhydrous potassium carbonate (3.36g, 24.32mmol) were added to a 100mL round bottom flask, 50mL of acetone was added, the temperature was slowly raised to 52°C, and 1,2-dibromo Ethane (3.02mL, 34.5mmol), continue to react for 75h after dropping, filter the reaction solution, collect the filtrate, distill the solvent under reduced pressure at 40°C, and then separate and purify by silica gel column chromatography. The eluent is petroleum ether and Ethyl acetate, using gradient elution, the volume ratio of petroleum ether / ethyl acetate gradually increased from 1:2 to 1:4, and dried under vacuum at 40°C to obtain white solid N9-bromoethyl-O 6 - Benzylguanine (2.16 g, 6.22 mmol), yield 71%.
[0103] UVλ:248,283...
Embodiment 3
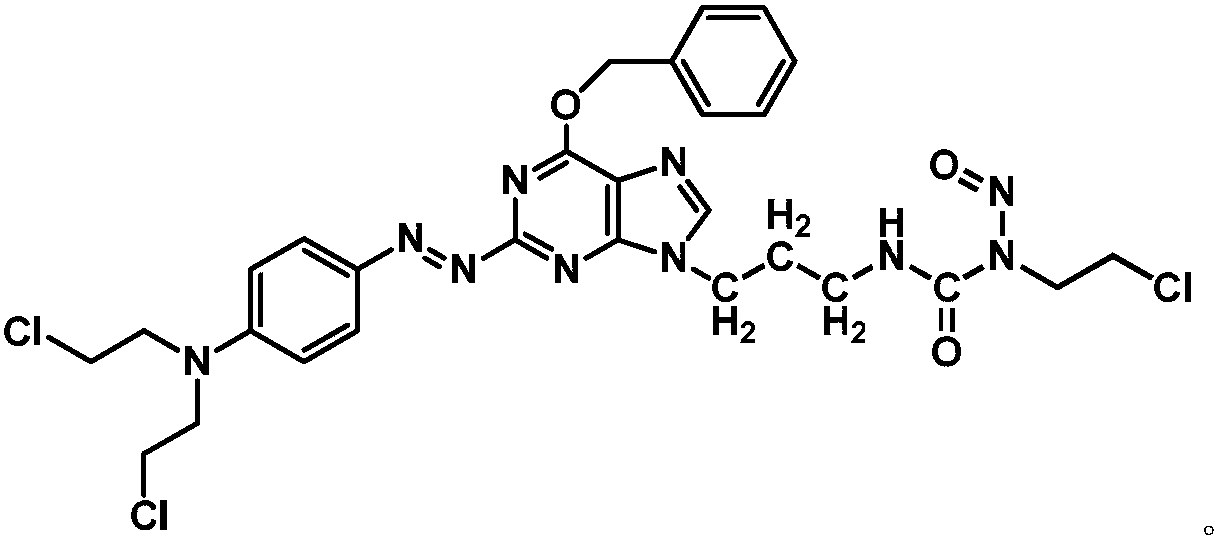
[0143] Example 3: (E)-3-(3-(6-(benzyloxy)-2-((4-bis(2-chloroethyl)amino)phenyl)diazenyl)-9H-purine Synthesis of -9-yl)propyl)-1-(2-chloroethyl)-1-nitrosourea (Compound 2)
[0144] 1) N9-bromopropyl-O 6 -Synthesis of benzylguanine
[0145] Weigh O 6 - Benzylguanine (1.45g, 6mmol), anhydrous potassium carbonate (2.76g, 20mmol) were added to a 50mL round bottom flask, 50mL of acetone was added, the temperature was slowly raised to 55°C, and 1,3 dibromopropane (3.03 mL, 30mmol), continue to react for 65h after dropping, filter the reaction solution, collect the filtrate, and distill off the solvent under reduced pressure at 45°C, then use silica gel column chromatography to separate and purify, the eluent is petroleum ether and ethyl acetate, using a gradient Elution, the volume ratio of petroleum ether / ethyl acetate gradually increased from 1:2 to 1:4, and vacuum drying at 40 °C gave white solid N9-bromopropyl-O 6 - Benzylguanine (1.41 g, 3.9 mmol), yield 65%.
[0146] UVλ:2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com