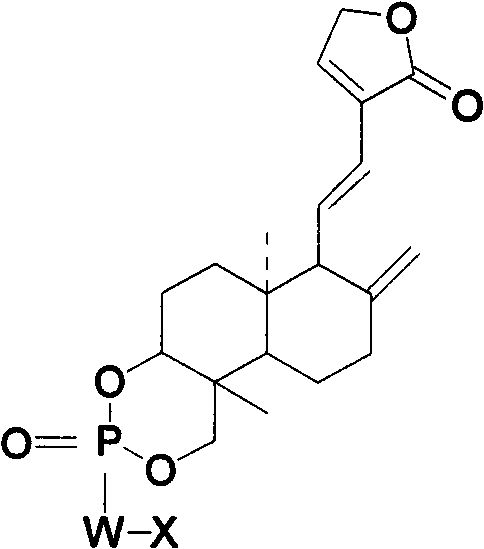
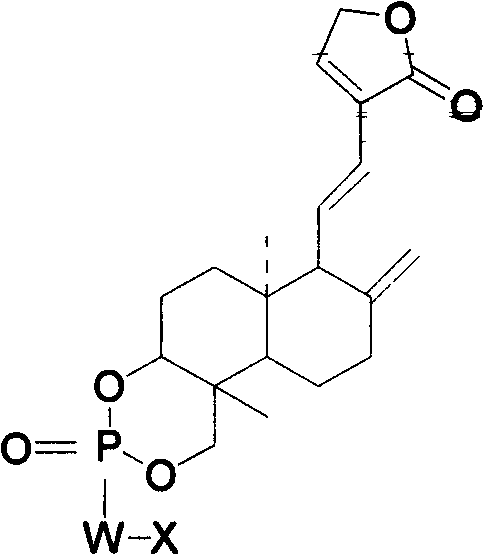
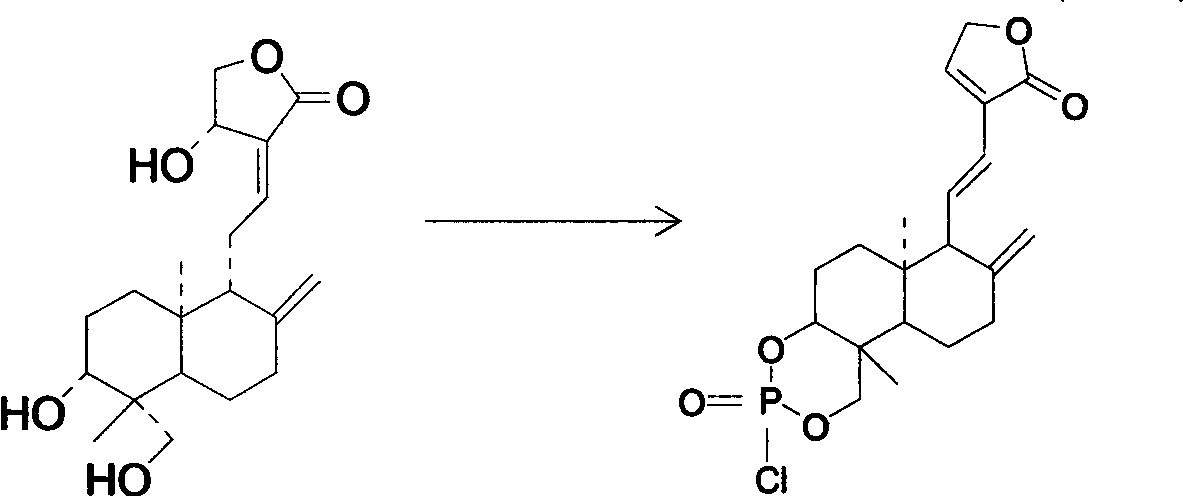
Andrographolide phosphoric acid derivatives and preparation method thereof
A technology of andrographolide and andrographolide, applied in the field of andrographolide phosphoric acid derivatives and its preparation, can solve the problems of unsatisfactory solubility, low bioavailability, and large irritation, and achieve good antiviral infection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1, the preparation method of andrographolide phosphorus oxychloride:
[0024] 20g of dehydroandrographolide was dissolved in 80ml of pyridine and cooled. Add POCl dropwise at 0°C 3 30g (dissolved in 50ml chloroform), then reacted for 0.5h, with saturated Na 2 CO 3 , water were washed separately, anhydrous MgSO 4 Drying, draining, and ethanol recrystallization gave 18 g of colorless dehydroandrographolide phosphorus oxychloride with a yield of 90%.
[0025]
Embodiment 2
[0026] Embodiment 2, the preparation method of andrographolide phosphorus oxychloride:
[0027] Dissolve 10g of andrographolide in 40ml of pyridine, add 120ml of chloroform at the same time, and cool with cold salt. At -5°C, POCl was added dropwise 3 / CHCl 3 liquid (containing POCl 3 18g), reaction 1h, with saturated Na 2 CO 3 , water were washed separately, anhydrous MgSO 4 Drying, vacuuming, and recrystallization from methanol gave 9 g of colorless andrographolide phosphorus oxychloride with a yield of 90%.
Embodiment 3
[0028] Embodiment 3, the preparation method of andrographolide phosphoric acid:
[0029] Dissolve 35g (0.1mol) of andrographolide in 150ml of pyridine, cool, and at -10°C, add POCl dropwise at a controlled rate 3 65g (0.4mol), stirred at 0°C for 0.5h, added saturated Na 2 CO 3 The pH of the solution was adjusted to 7 (at this time, the temperature was controlled at 3°C). And a small amount of acetone to dissolve the flocculent precipitate, then stir and hydrolyze for 40h at 20°C.
[0030] The organic phase was extracted three times with 60 ml of chloroform, the aqueous layer was adjusted to pH=3 with hydrochloric acid, the precipitate was completely precipitated, and suction filtered to obtain 29.7 g of andrographolide phosphate as a light blue solid (yield 85%)
[0031]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com