Nitrogen mustard quercetin derivative, and preparation method and application thereof
A technology of quercetin and its derivatives, which is applied in the field of medicine and can solve problems such as the toxicity of metabolites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of Nitrogen Mustard Quercetin Derivatives (1)
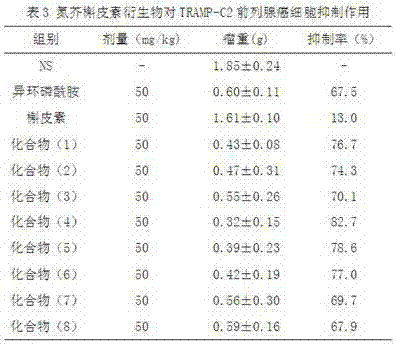
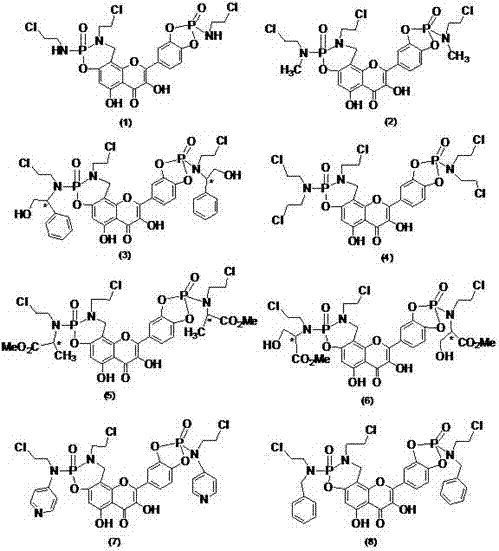
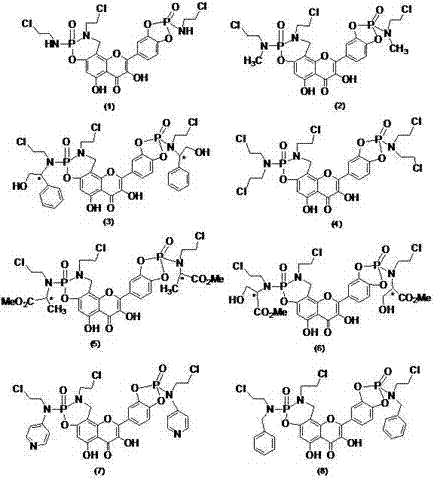
[0020] N 2 Under protection, add 97.6mg (1.2mmol) of formaldehyde solution, 94.8mg (1.2mmol) of 2-chloroethylamine, 302mg (1.0mmol) of quercetin and 5 mL of ethanol into the closed reactor, add 4 drops of hydrochloric acid, Heat to 75-80°C for 3 hours, cool naturally, precipitate solid, filter with suction, and separate the crude product by chromatography (V (n-butanol): V (water): V (HOAc) = 4:1:1, wash with methanol Removed to obtain quercetin Mannich base yellow solid 129mg, yield 33.0%.
[0021] Take 393mg (1mmol) of the above-mentioned Mannich base, 431mg (2.2mmol) of 2-chloroethylphosphoryl dichloride, dissolve it with 5mL of dry DMF, and add 0.6mL of Et 3 The mixture of N and 4mL dry DMF was dripped over 30min, reacted at room temperature for 2h, then heated to 60°C, and the reaction was complete as monitored by TLC. Adjust the pH of the solution to 3-4 with 10% HCl solution, and then add 50mL of water...
Embodiment 2
[0023] Preparation of nitrogen mustard derivatives of quercetin (2)
[0024] Replace 431mg (2.2mmol) 2-chloroethylphosphoryl dichloride in Example 1 with 462mg (2.2mmol) (N-2-chloroethyl-N'-methyl) phosphoryl dichloride, other operations are the same as The same as in Example 1, the quercetin derivative (2) was obtained with a yield of 72.5%. MS (EIS)[M+H] + = 668.0.
Embodiment 3
[0026] Preparation of Nitrogen Mustard Quercetin Derivatives (3)
[0027] Replace 431mg (2.2mmol) of 2-chloroethylphosphoryl dichloride in Example 1 with 696mg (2.2mmol) L-(N-2-chloroethyl-N'-hydroxymethylbenzyl) phosphoryl dichloride Chlorine, other operations were the same as in Example 1 to obtain a quercetin derivative (3) with a yield of 71%. MS (EIS) [M+H] + = 880.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com