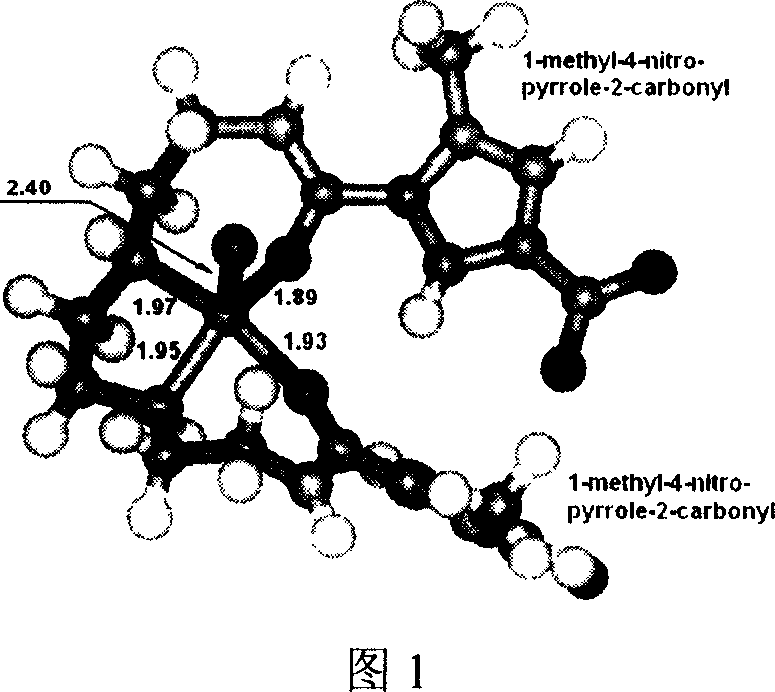
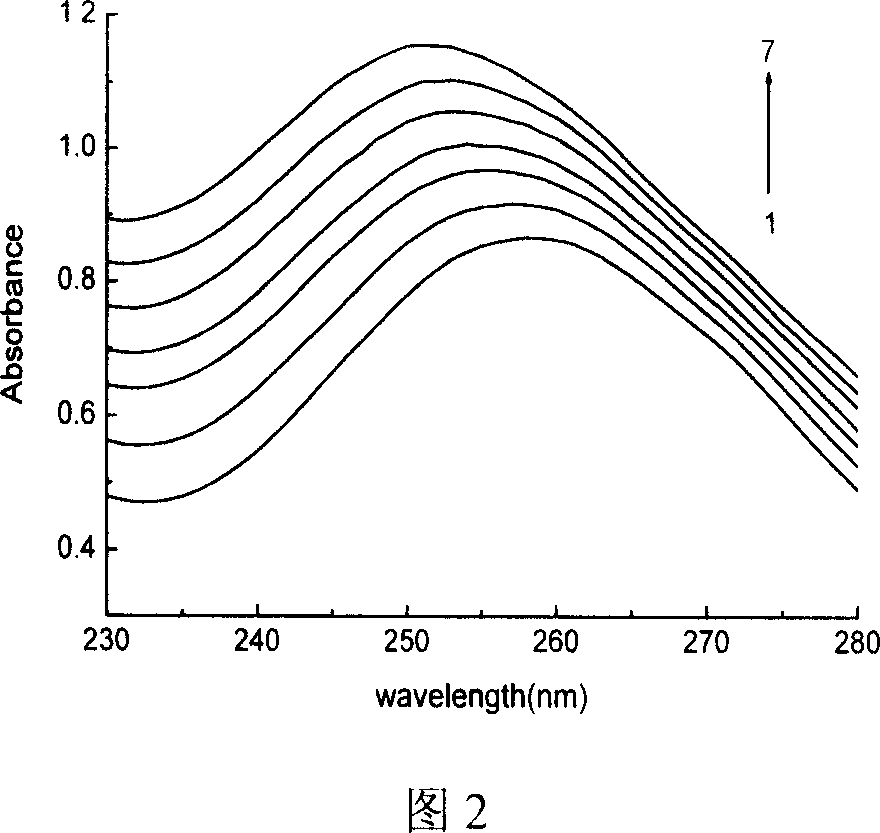
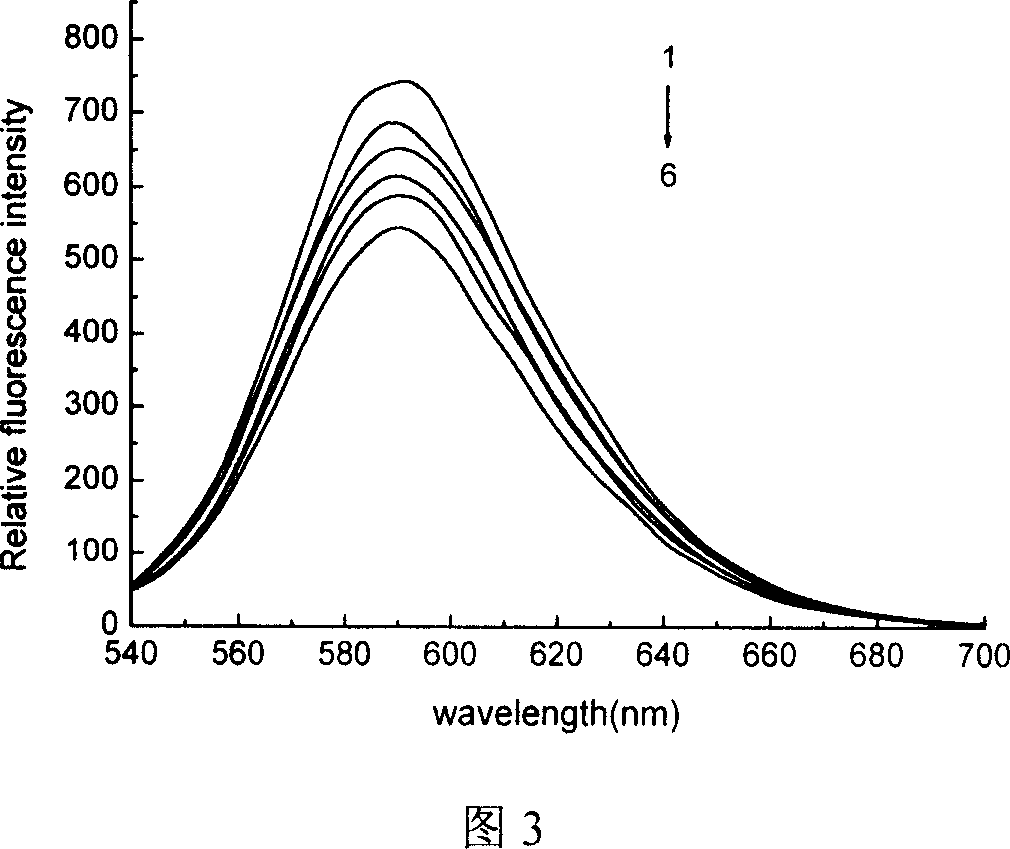
N1,N8-disubstituent-triethyl tetramine copper (II) complex and its prepn process
A technology of triethylenetetramine and di-substitution, which is applied in the direction of copper organic compounds, medical preparations containing active ingredients, and pharmaceutical formulas. It can solve problems such as research difficulties, low content, and complex structures, and achieve a simple and easy synthesis method. line, easy purification, and high-yield results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] N 1 , N 8 -Synthesis of bis(1-methyl-4-nitropyrrole-2-yl)triethyltetramine copper(II) complex
[0088] Under stirring, dissolve 10.02g (36.9mmol) N-methyl-2-trichloroacetyl-4-nitropyrrole in 60mL of DMF, and cool at 0°C for 20min; under stirring, dissolve 2.70g (18.5mmol) 20mL of triethylenetetramine in DMF was added dropwise to the above solution within 30min; after the dropwise addition, the reaction mixture was stirred at 0°C for 2h, then raised to room temperature and continued to stir for 2h. Stop the reaction, add 200mL of water to the mixture, precipitate a yellow precipitate, filter, wash the precipitate with THF 3 times, 10mL each time, recrystallize the resulting solid with hot THF, and dry in vacuo to obtain 5.84gN 1 , N 8 -Bis(1-methyl-4-nitropyrrole-2-yl)triethyltetramine yellow solid (structural formula is as follows), the yield is 77%.
[0089]
[0090] The obtained yellow solid was analyzed and characterized:
[0091] 1. The measured melting poin...
Embodiment 2
[0105] N 1 , N 8 -Synthesis of bis(1-ethyl-4-nitropyrrole-2-yl)triethyltetramine copper(II) complex
[0106] Under stirring, dissolve 10.86g (38.0mmol) N-ethyl-2-trichloroacetyl-4-nitropyrrole in 60mL of DMF, and cool at 0°C for 20min; under stirring, dissolve 2.77g (19.0 mmol) 20 mL of triethylenetetramine in DMF was added dropwise to the above solution within 30 min; after the dropwise addition, the reaction mixture was stirred at 0°C for 2 h, then raised to room temperature and continued to stir for 2 h. Stop the reaction, add 200mL of water to the mixture, precipitate a yellow precipitate, filter, wash the precipitate with THF 3 times, 10mL each time, recrystallize the resulting solid with hot THF, and dry in vacuo to obtain N 1 , N 8 - bis(1-ethyl-4-nitropyrrole-2-yl)triethyltetramine yellow solid.
[0107] 0.405g (0.846mmol) N 1 , N 8 -bis(1-ethyl-4-nitropyrrole-2-yl)triethylenetetramine was dissolved in 100mL of DMF to form a ligand solution; 0.1443g (0.846mmol) C...
Embodiment 3
[0109] N 1 , N 8 -Synthesis of bis(1-propyl-4-nitropyrrole-2-yl)triethyltetramine copper(II) complex
[0110] Under stirring, dissolve 11.56g (38.6mmol) N-propyl-2-trichloroacetyl-4-nitropyrrole in 60mL of DMF, and cool at 0°C for 20min; under stirring, dissolve 2.82g (19.3 mmol) 20 mL of triethylenetetramine in DMF was added dropwise to the above solution within 30 min; after the dropwise addition, the reaction mixture was stirred at 0°C for 2 h, then raised to room temperature and continued to stir for 2 h. Stop the reaction, add 200mL of water to the mixture, precipitate a yellow precipitate, filter, wash the precipitate with THF 3 times, 10mL each time, recrystallize the resulting solid with hot THF, and dry in vacuo to obtain N 1 , N 8 - bis(1-propyl-4-nitropyrrole-2-yl)triethyltetramine yellow solid.
[0111] 0.394g (0.778mmol) N 1 , N 8 -bis(1-propyl-4-nitropyrrole-2-yl)triethyltetramine was dissolved in 100mL of DMF to form a ligand solution; 0.1327g (0.778mmol) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com