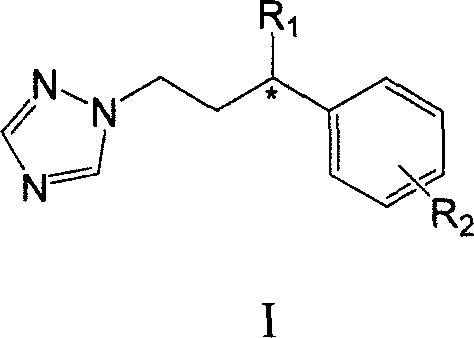
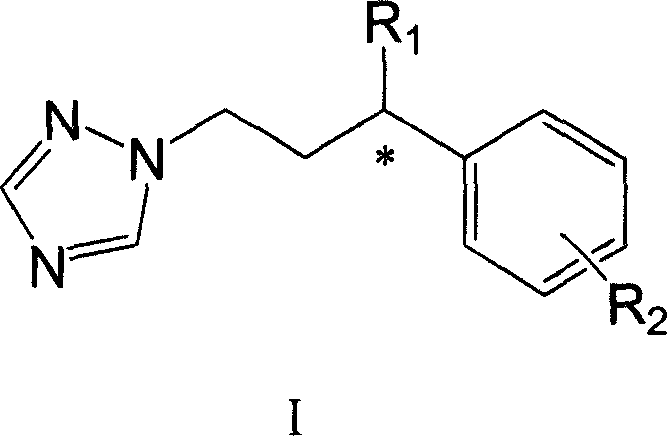
Gamma-aryl substituted chiral triazole bactericides and their synthesis process
A triazole and chiral technology, applied in the field of chiral triazole compounds and their synthesis, can solve problems such as rare reports, achieve simple separation and purification, good product yield and enantioselectivity, and simple and easy synthesis method row effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Under nitrogen protection, 20 ml of anhydrous tetrahydrofuran solution of D-(-)-camphorsultam (1a) (10.76 g, 50 mmol) was added dropwise to 40 ml of sodium hydride (2.4 g, 100 mmol). mL of anhydrous tetrahydrofuran solution, react at room temperature for 1 hour, then drop cinnamoyl chloride (10.0 g, 60 mmol) in 20 mL of tetrahydrofuran solution into the reaction flask. After reacting at room temperature for 24 hours, 20 ml of ice water was added to terminate the reaction, extracted with diethyl ether (3×40 ml), the organic phase was washed with water, and anhydrous MgSO 4 Drying, concentration under reduced pressure, recrystallization from a mixed solution of ethyl acetate and petroleum ether (volume ratio 1:1) yielded 15.75 g of white solid product 2a, with a yield of 91.2%. 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 0.99 (s, 3H), 1.21 (s, 3H), 1.36-1.48 (m, 2H), 1.91-1.93 (m, 3H), 2.12-2.21 (m, 2H), 3.49 (d , J=14.0Hz, 1H), 3.56(d, J=14.0Hz, 1H), 4.0(dd, 1H), 7.17(d, J=...
Embodiment 2
[0033] Except that L-(+)-camphorsultam (1b) was used instead of D-(-)-camsylsultam, other conditions were the same as in Example 1. The obtained 2b, 3b, 4b, 5b, 6b are as follows:
[0034] 2b: 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 0.99 (s, 3H), 1.21 (s, 3H), 1.36-1.48 (m, 2H), 1.91-1.93 (m, 3H), 2.12-2.21 (m, 2H), 3.49 (d , J=14.0Hz, 1H), 3.56(d, J=14.0Hz, 1H), 4.0(dd, 1H), 7.17(d, J=15.2Hz, 1H), 7.38-7.43(m, 3H), 7.56 -7.60(m, 2H), 7.80(d, J=15.2Hz, 1H).
[0035] 3b: 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 0.81 (t, 3H), 0.96 (s, 3H), 1.17 (s, 3H), 1.12-1.35 (m, 6H), 1.59-1.65 (m, 2H), 1.84-1.88 (m , 3H), 2.01(m, 2H), 3.06(m, 2H), 3.22(m, 1H), 3.40(d, J=14.0Hz, 1H), 3.48(d, J=13.6Hz, 1H), 3.80 (dd, 1H), 7.19(m, 3H), 7.27(m, 2H).
[0036] 4b: 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 0.82 (t, 3H), 1.10-1.29 (m, 4H), 1.57-1.64 (m, 2H), 1.81 (m, 2H), 1.92-1.97 (m, 1H), 2.67 (m , 1H), 3.45-3.53 (m, 2H), 7.17 (m, 3H), 7.29 (m, 2H).
[0037] 5b: 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 0.8...
Embodiment 3
[0040] (1) Raw material, operation and condition are the same as embodiment 1 step (1).
[0041] (2) Under the protection of nitrogen, add compound 2a (3.45 g, 10 mmol) and 50 ml of anhydrous tetrahydrofuran into a dry three-necked flask, and add the freshly prepared ether solution of n-EtMgBr (24 mmol 20 ml), after the dropwise addition was completed, the stirring reaction was continued at -78°C for 3 hours, the temperature was raised to -40°C, and the reaction was terminated with saturated aqueous ammonium chloride solution, extracted with ether, and the organic phase was washed with saturated brine, anhydrous MgSO 4 It was dried, concentrated under reduced pressure, and separated by silica gel column chromatography to obtain 1.97 g of the product N-(3'R)-3'-phenylpentanoylcamphorane-10,2-sultam (3a), with a yield of 52.5%. 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 0.78 (t, 3H), 0.96 (s, 3H), 1.17 (s, 3H), 1.31-1.36 (m, 2H), 1.57-1.69 (m, 2H), 1.84-1.89 (m , 3H), 2.01(m, 2H), 2.9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com