Preparation method and application of novel ferrocene derivative containing free radicals of nitroxide
A technology of ferrocene derivatives and nitroxide free radicals, applied in the field of medicine, can solve the problems of poor overall clinical treatment effect and difficult cancer treatment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
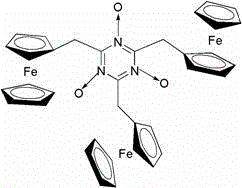
[0047] Embodiment 1 Synthesis of ferrocene derivatives I containing nitrogen and oxygen free radicals
[0048] Add cyanuric chloride (TCT) (0.184g, 1 mmol) and 30 mL of dry tetrahydrofuran into a 100 mL round-bottomed flask, stir in an ice bath, and dry 20 mL of ferrocenemethanol (0.864 g, 4 mmol) THF solution was slowly added dropwise to the reaction system. After stirring in an ice bath for 30 minutes, slowly add 10 mL of dry tetrahydrofuran solution containing DMAP (0.366 g, 3 mmol) into the reaction system dropwise, stir in an ice bath for 30 minutes, then naturally rise to room temperature for reaction, and react at room temperature for 5-6 hours , followed by reflux reaction, after the completion of the TLC detection reaction, the reaction solution was concentrated under reduced pressure, and the residue was directly separated by column, and used ( V 石油醚 : V 乙酸乙酯 , 5:1-2:1) mobile phase elution to obtain ferrocene derivative I containing nitroxide free radicals, 0.5...
Embodiment 2
[0050] Example 2 Single crystal cultivation and XRD characterization
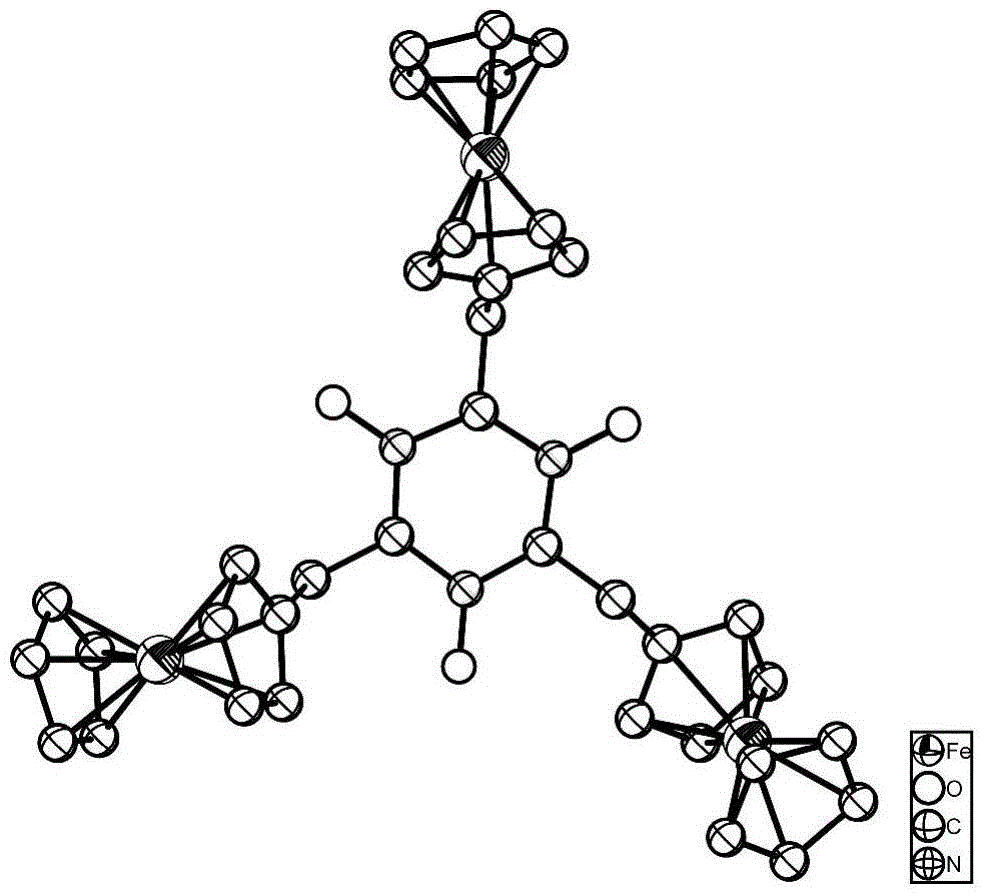
[0051] Take a small amount of ferrocene derivatives containing nitrogen and oxygen free radicals obtained in Example 1, dissolve them in a mixed solution of ethyl acetate / petroleum ether (V / V, 1:4), and naturally volatilize and crystallize at room temperature to obtain light yellow Needle crystals. The obtained crystals were characterized by XRD, the space group is P-1, and the unit cell parameters are a =13.8909?, b=15.1596?, c=16.1133?; α= 74.027 o , β=77.179 o , γ=80.454 o ; V=3160.88 ? 3 , Z=4, R1=0.0437.
Embodiment 3
[0052] Example 3 Anticancer Activity Experiment
[0053] MTT method was used to screen the activity of human lung cancer cell line (A549) and breast cancer cell line MCF-7.
[0054] The specific experimental operation process is as follows:
[0055] (1) The colorectal cancer cell line A549 in the logarithmic growth phase was inoculated in a 96-well culture plate and cultured. When the cells grew to 90%, 1 μL of 1×10 -4 The test sample of M was added, and its concentration was set to 3 duplicate wells, and a positive control (gefitinib) was set, and a negative control was 1 μL (DMSO+cell culture solution+MTT). Then at 37℃, 5%CO 2 Cultivate under conditions for 48 hours; (2) Then add 20 μL of 5mg / mL MTT solution to each well, shake gently and continue for several minutes, then continue to cultivate for 3-4 hours, then suck out the culture solution; (3) Each well Add 100 μL of DMSO and shake on a shaker at low speed for 10 minutes to fully dissolve the crystals. Meas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com