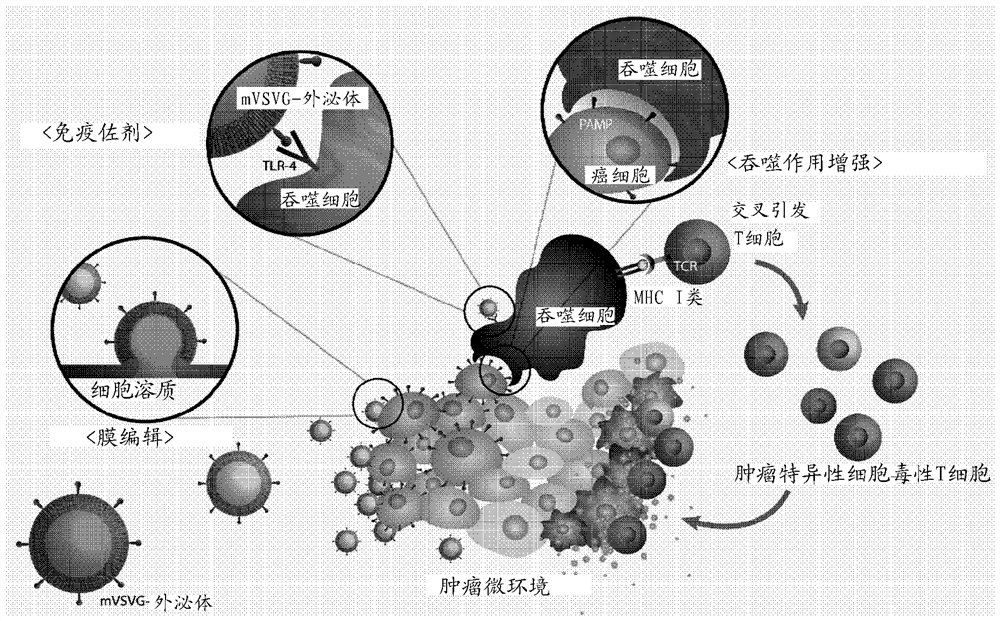
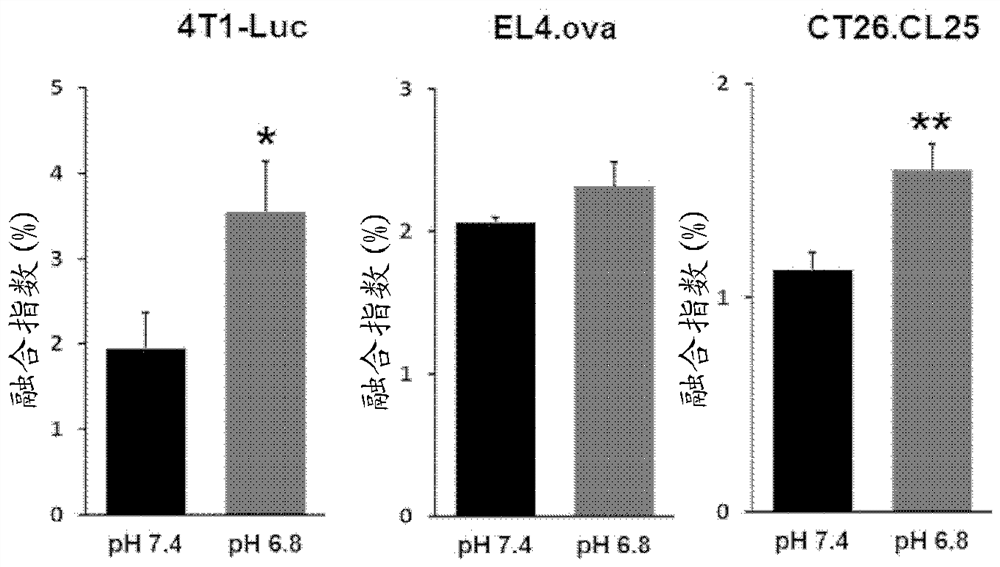
Novel recombinant plasma membrane-based vesicle, for treating cancer
A technology of vesicles and plasma membranes, applied in the field of vesicles based on recombinant plasma membranes, which can solve the problems of ineffective killing of cancer cells and difficulties in the supply of neural stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1: Preparation of VSV-G H162R construct
[0083] In order to prepare recombinant exosomes including the VSV-G H162R mutein, the inventors first prepared a gene construct encoding the H162R mutein, in which the 162nd amino acid residue of VSV-G (based on the protein disclosed in GenBank number CAC47944 No. 178 of ), the composition is replaced by arginine. Specifically, for this purpose, site-directed mutagenesis was performed using a plasmid DNA (pCMV-VSV-G envelope vector, RV-110, Cell Biolabs, hereinafter abbreviated as "VSV-G construct") that included as a template The polynucleotide (SEQ ID NO: 2) encoding the wild-type VSV-G protein (SEQ ID NO: 1), together with the forward primer of SEQ ID NO: 3 (5'-ATA TGC CCC ACT GTC CGC AAC TCT ACA ACC TGG-3') and the reverse primer of SEQ ID NO: 4 (3'-TAT ACG GGG TGA CAG GCG TTG AGA TGT TGG ACC-5') (bold letters correspond to the mutated amino acid arginine).
Embodiment 2
[0084] Example 2: Preparation of recombinant exosomes including VSV-G H162R
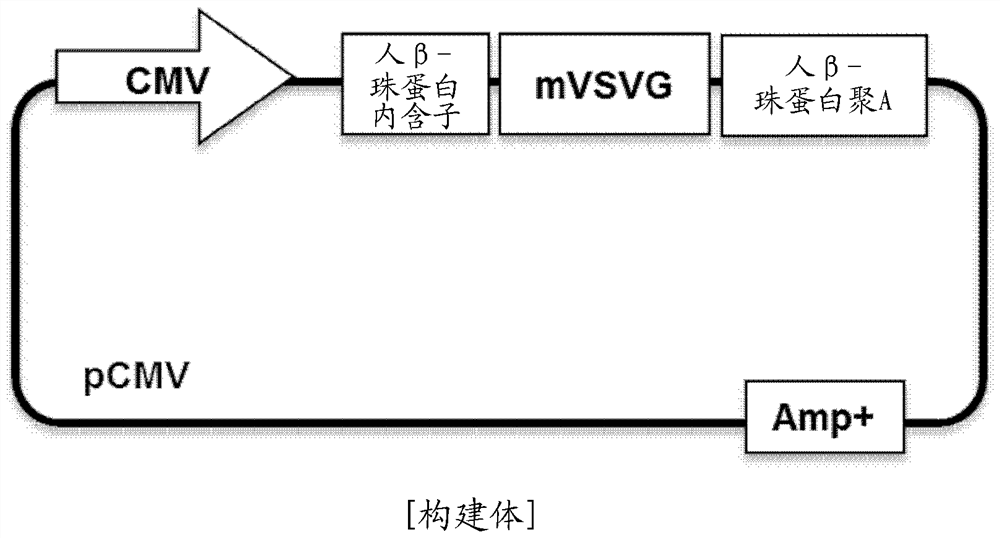
[0085] The inventors made the pCMV-VSV-G H162R plasmid vector (hereinafter abbreviated as "VSV-G H162R construct", Figure 2a and 2b ) was transfected into HEK293T cells and cultured for 48 hours, and the gene encoding the VSV-G H162R protein (SEQ ID NO: 5) prepared in Example 1 above was inserted into the pCMV-VSV-G H162R plasmid vector (SEQ ID NO: 6). Subsequently, the cell culture was recovered, sequentially centrifuged at 300 g for 10 minutes, 2,000 g for 10 minutes, and 10,000 g for 30 minutes, filtered using a 0.2 μm filter, and recovered by ultrafiltration at 150,000 g for 3 hours clumps ( image 3 ).
[0086] Subsequently, in order to confirm whether the VSV-G H162R mutant protein was included in the exosomes, a fraction of the recovered exosomes was disrupted, and an anti-VSV-G antibody (Abcam, ab50549) and an anti-Alix antibody (exosome marker, Santacruz, sc99010) performed Western b...
Embodiment 3
[0090] Example 3: Preparation of recombinant exosomes in which doxorubicin is encapsulated
[0091] In order to load doxorubicin (DOX), an immunogenic cell death inducer, into the recombinant exosomes prepared in Example 2 above, the purified exosomes (approximately 10 11 exosomes) were mixed with DOX in 1 mL PBS. Subsequently, the DOX recombinant exosome mixture was sonicated using a Model 505 Sonic Dismembrator with a 0.25-inch tip at the following settings: 20% amplitude, 6 cycles of on / off 30 seconds for 3 minutes, with 2 minute cool down period between cycles. After sonication, the Exo-DOX solution was incubated at 37 °C for 60 min to recover exosome membranes. Excess free drug was removed by size exclusion chromatography using a NAP-10 Sephadex G25 column (GE Healthcare, Buckinghamshire, UK).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com