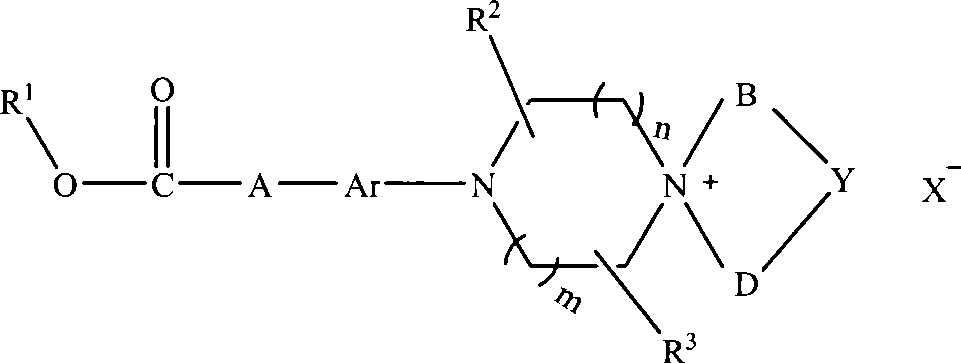
Aromatic chlorethazine piperazine quaternary ammonium salt derivatives, and preparation and use thereof
A technology of aromatic nitrogen mustard and quaternary ammonium salt, which is applied in the direction of drug combination, pharmaceutical formula, medical preparations containing active ingredients, etc., and can solve problems such as limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0097] Preparation of γ-(p-aminophenyl)butyric acid
[0098]
[0099] β-(4-Acetamidobenzoyl)-propionic acid (89g, 378mmol), potassium hydroxide (88g, 1.57mol), 65ml of 85% hydrazine hydrate and 450ml of diethylene glycol were heated to reflux (about 140°C) for 1.5 hours , remove the condensing tube to raise the temperature to 195°C, steam off excess hydrazine hydrate, and keep the temperature to continue reflux for 4 hours. After cooling to room temperature, the reaction system was diluted with about 400ml of water, and the pH was adjusted to neutrality with 6N hydrochloric acid. Crystals were precipitated, filtered, and washed with water to obtain 44 g of white crystals, with a yield of 65.0%. -120°C).
[0100] 1 H NMR (DMSO, 300MHz): 11.2-12.3 (b, 1H, COO H), 6.81 (d, 2H, J=8.0Hz, ArH), 6.47 (d, 2H, J=8.0Hz, ArH), 4.60-5.70 (bs, 2H, NH 2 ), 2.38(t, 2H, J=7.4Hz, Ph- CH 2 ), 2.15(t, 2H, J=7.4Hz, CH 2 -COOH), 1.68(m, 2H, Ph-CH 2 - CH 2 ).
[0101] Pre...
preparation Embodiment 1
[0131] Preparation Example 1 Preparation of Chlorinated 4-(4'-ethoxycarbonylpropylphenyl)-1,1-dimethyl-1,4-diazacyclohexane
[0132]
[0133] The mixed solution of CLBM (2.0g, 6mmol), dimethylamine hydrochloride (0.6g, 7mmol), 40ml ethanol and sodium bicarbonate (5.0g, 59mmol) was stirred and heated to reflux for 24 hours, and TLC showed that most of the raw materials had been After the reaction, excess sodium bicarbonate was removed by filtration, the filtrate was evaporated to dryness under reduced pressure, dissolved in 20ml of water, extracted twice with ethyl acetate (15ml×2), the water layer was evaporated to dryness under reduced pressure, and recrystallized from ethanol-ethyl acetate to obtain 1.4 g white needle crystals, yield 68.2%, Mp: 230-237°C.
[0134] 1 H NMR (D 2 O, 300MHz): 7.09(d, 2H, J=8.7Hz, ArH), 6.90(d, 2H, J=8.7Hz, ArH), 3.87(q, 2H, J=6.9Hz, CH 2 -CH 3 ), 3.43-3.47 (m, 4H, N- CH 2 ), 3.35-3.37 (m, 4H, N + - CH 2 ), 3.08(s, 6H, N + ...
preparation Embodiment 2
[0136] Preparation Example 2 Preparation of 2,4-dimethyl-9-(4'-ethoxycarbonpropylphenyl)-3-oxa-6,9-diazaspiro[5.5]undecane chloride
[0137]
[0138] The preparation was carried out according to the method in Preparation Example 1, CLBM and 2,6-dimethylmorpholine were reacted in equimolar amounts, and white flaky crystals were obtained after post-treatment, with a yield of 76.2%, Mp: 276-278°C.
[0139] 1 H NMR (D 2 O, 300MHz): 7.09 (d, 2H, J = 8.7Hz, ArH), 6.89 (d, 2H, J = 8.7Hz, ArH), 4.13 (m, 2H, O- CH ), 3.87(q, 2H, J=7.2Hz, CH 2 -CH 3 ), 2.91-3.77 (m, 12H, N- CH 2 , N + - CH 2 ), 2.44(t, 2H, J=7.2Hz, Ph- CH 2 ), 2.18(t, J=7.2Hz, 2H, Ph-CH 2 -CH 2 - CH 2 ), 1.72-1.79 (m, 2H, Ph-CH 2 - CH 2 ), 1.09 (d, 6H, J=6.3Hz, O-CH- CH 3 ), 1.04(t, 3H, J=7.2Hz, CH 2 - CH 3 ).
[0140] Elemental Analysis: C 22 h 35 ClN 2 o 3 , measured: C, 64.10; H, 8.48; N, 6.60; theoretical: C, 64.29; H, 8.58; N, 6.82.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com