Comprehensive utilization method for laterite-nickel ore
A technology of laterite nickel ore and mixture, which is applied to magnesium and aluminum, comprehensively utilizes laterite nickel ore, can solve the problems of polluting the environment, restricting the application of high-pressure acid leaching, pollution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
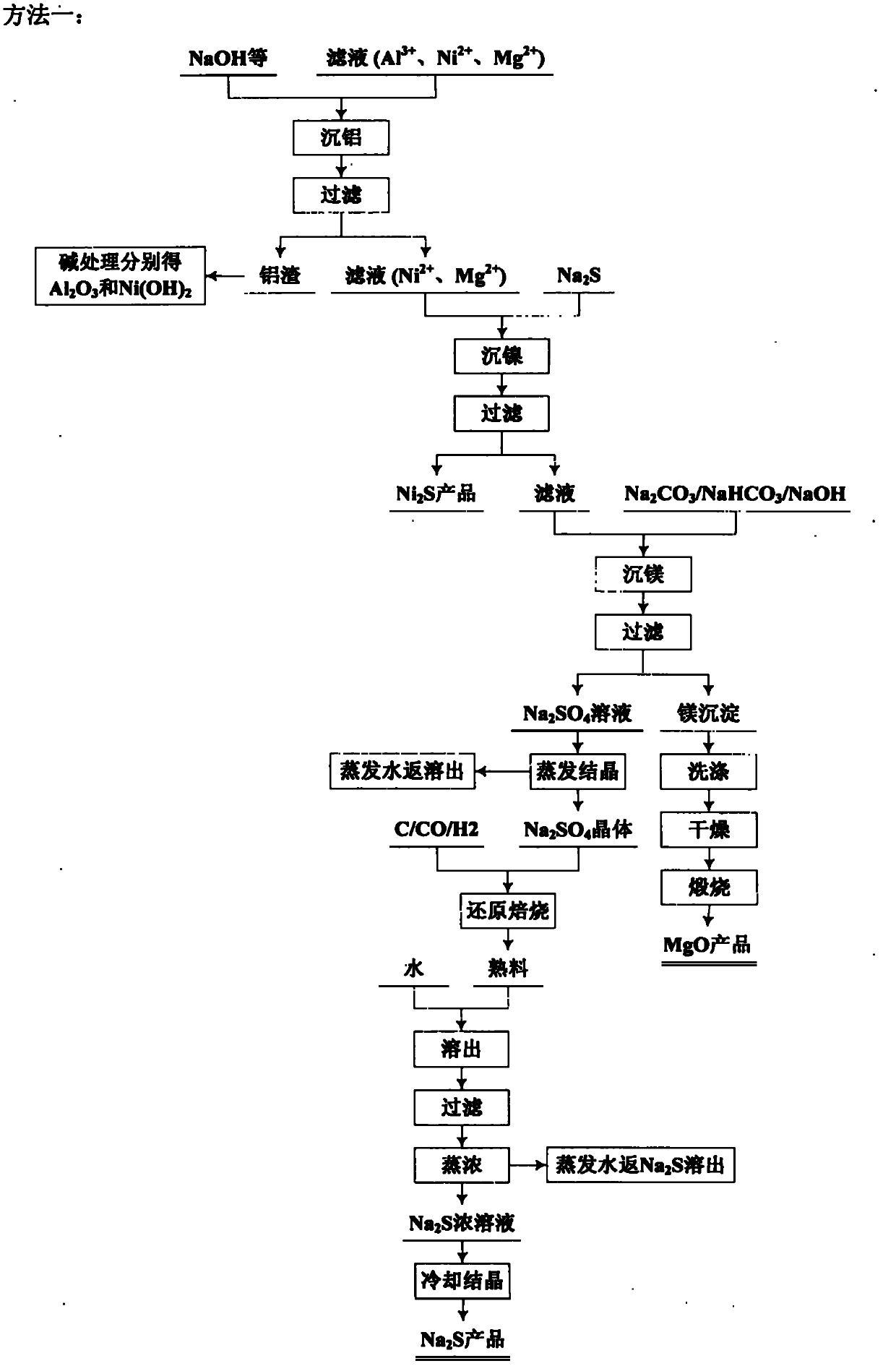
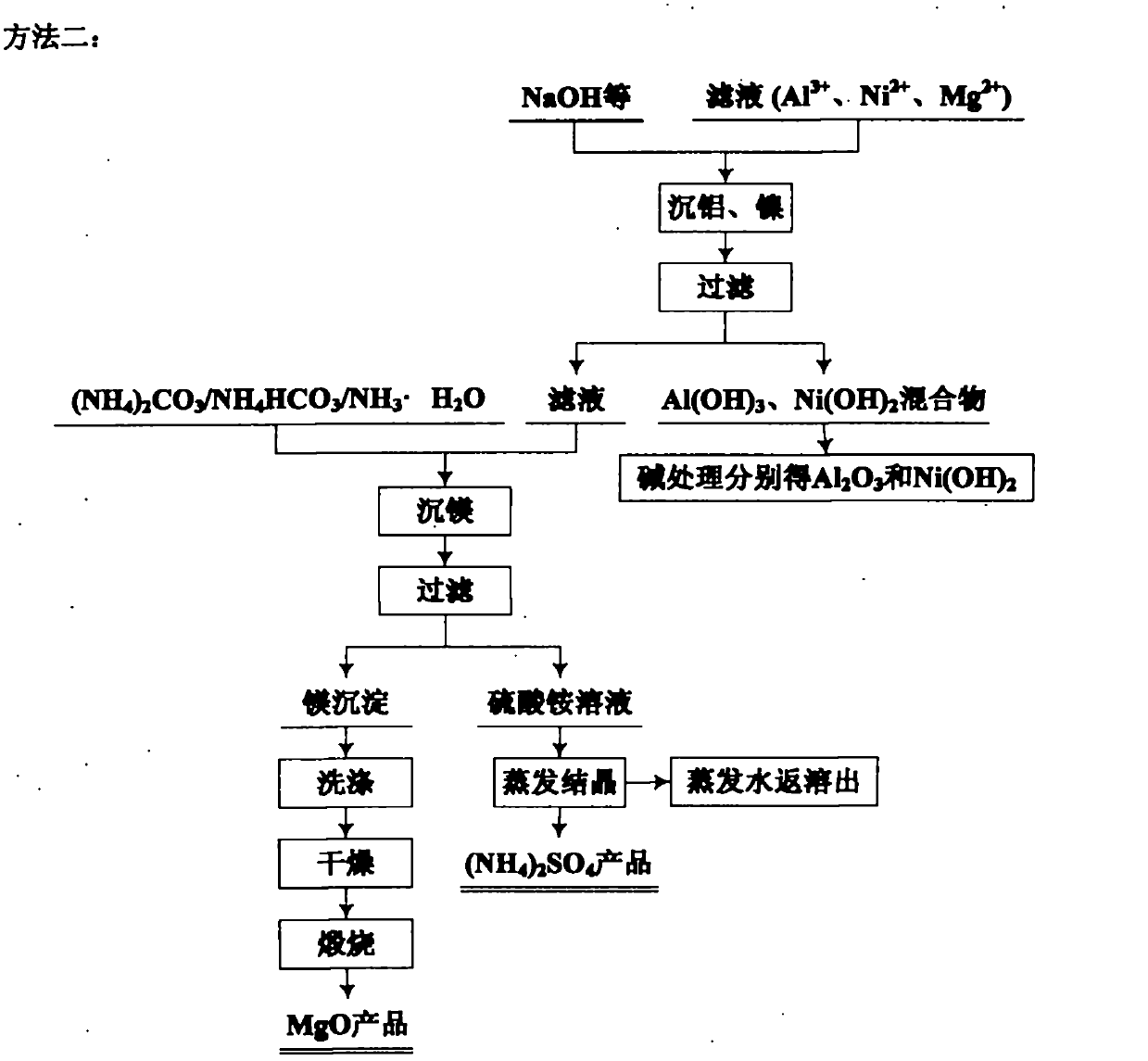
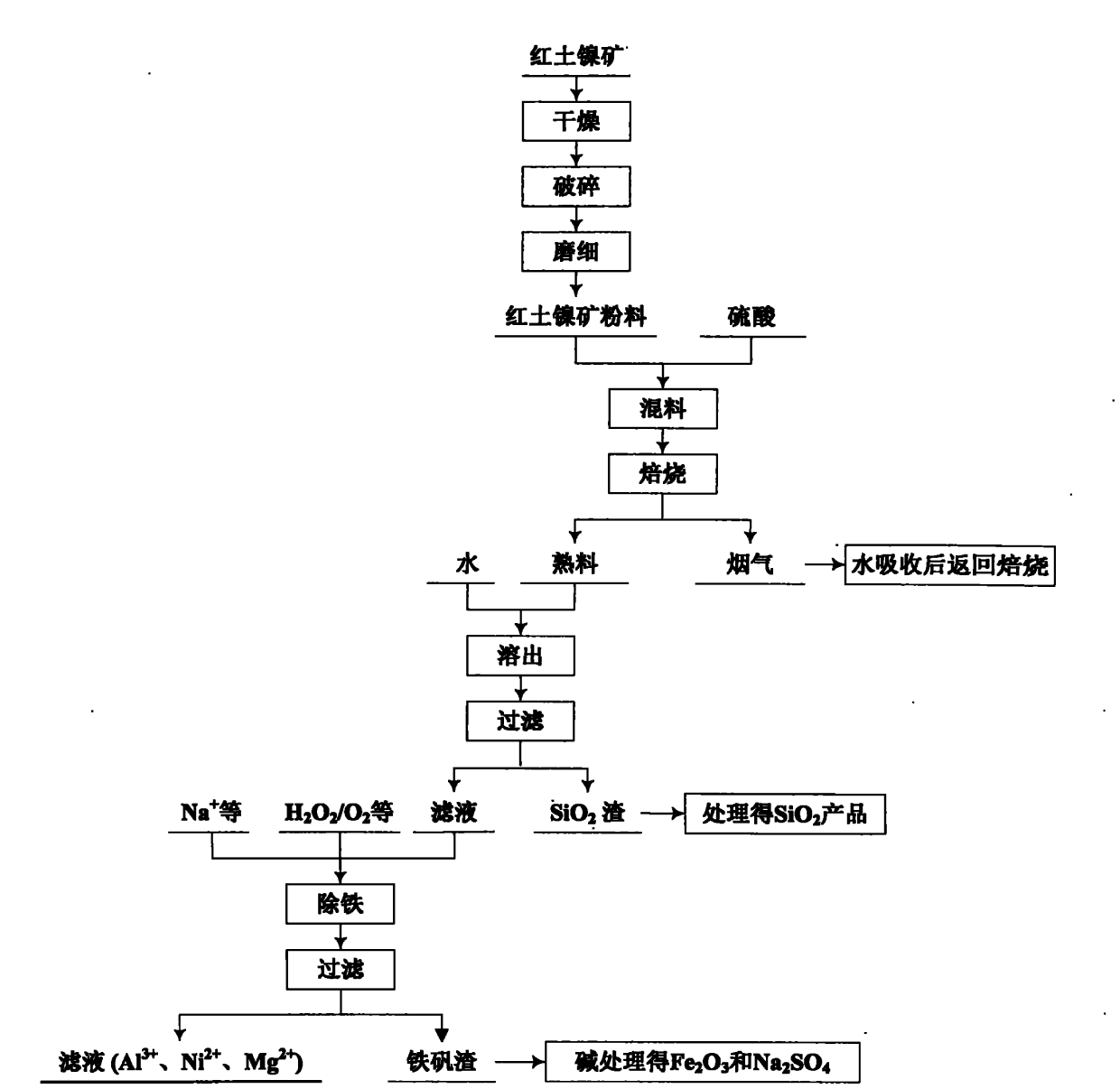
Image
Examples
Embodiment 1
[0101] The composition of the laterite nickel ore used is mainly Ni 0.71%, Fe 10.69%, Al 2 o 3 5.42%, MgO 28.09%, SiO 2 37.85%, loss on ignition 10.15%.
[0102] Crush the laterite nickel ore, grind it down to 80 μm, mix it with sulfuric acid with a concentration of 60% in a mass ratio of 1:1.1, raise the temperature to 300 ° C, keep the temperature for 2 hours, then raise the temperature to 360 ° C, and use the flue gas produced by the reaction Sulfuric acid is absorbed and returned for roasting.
[0103] Add the roasted clinker into water, dissolve it for 1 hour, and filter. The filter residue mainly contains silicon dioxide, which can be used as a product after further removal of impurities. The filtrate mainly contains Mg 2+ , Fe 3+ , Fe 2+ 、Ni 2+ 、Al 3+ , SO 4 2- . According to the Fe contained in the filtrate 2+ The amount of hydrogen peroxide was added in excess of 10% according to stoichiometry, and after reacting for 2 hours, sodium carbonate was added to ...
Embodiment 2
[0110] The lateritic nickel ore used is mainly composed of Ni 1.52%, Fe 11.02%, MgO 28.39%, Al 2 o 3 4.32%, SiO 2 38.13%, loss on ignition 11.21%.
[0111] Crush the laterite nickel ore, grind it down to 80 μm, mix it with 80% concentrated sulfuric acid at a mass ratio of 1:1.0, raise the temperature to 280°C, keep the temperature constant for 2.5 hours, then raise the temperature to 400°C, and use sulfuric acid to remove the flue gas produced by the reaction absorbed, and returned for roasting.
[0112] Add the roasted clinker into water, dissolve it for 1 hour, and filter. The filter residue mainly contains silicon dioxide, which can be used as a product after further removal of impurities. The filtrate mainly contains Mg 2+ , Fe 3+ , Fe 2+ 、Ni 2+ 、Al 3+ , SO 4 2- . According to the Fe contained in the filtrate 2+ The amount of hydrogen peroxide was added according to stoichiometric excess of 10%. After 2 hours of reaction, sodium bicarbonate was added to the sol...
Embodiment 3
[0118] The laterite nickel ore used mainly consists of Ni 2.01%, Fe 11.17%, Al 2 o 3 4.97%, MgO 32.83%, SiO 2 29.18%, loss on ignition 10.45%.
[0119] Crush the lateritic nickel ore, grind it to below 80 μm, mix it with sulfuric acid with a concentration of 70% in a mass ratio of 1:1.3, raise the temperature to 260 ° C, keep the temperature for 3 hours, then raise the temperature to 450 ° C, and use the flue gas produced by the reaction Sulfuric acid is absorbed and returned for roasting.
[0120]Add the roasted clinker into water, dissolve it for 2 hours, filter, the filter residue mainly contains silicon dioxide, and use it as a product after treatment. The filtrate mainly contains Mg 2+ , Fe 3+ , Fe 2+ 、Ni 2+ 、Al 3+ , SO 4 2- . According to the Fe contained in the solution 2+ Add sodium chlorate in excess of 5% according to the stoichiometric amount, and after reacting for 2 hours, add sodium hydroxide to the solution, control the temperature to 90° C., and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com