Tofacitinib crystal form compound and preparation method thereof
A technology of tofacitinib and citric acid, applied in the field of medicine, can solve the problems of inability to meet the requirements of active ingredients, unstable crystal particles, large crystal particle size, etc., so as to improve the quality of drug production and improve the bioavailability. , the effect of good liquidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
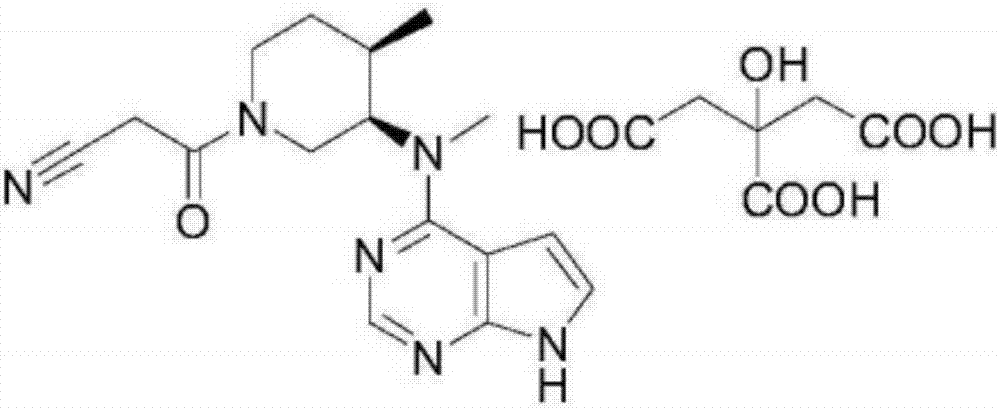
[0039] Embodiment 1: Preparation of tofacitinib citrate crystal compound
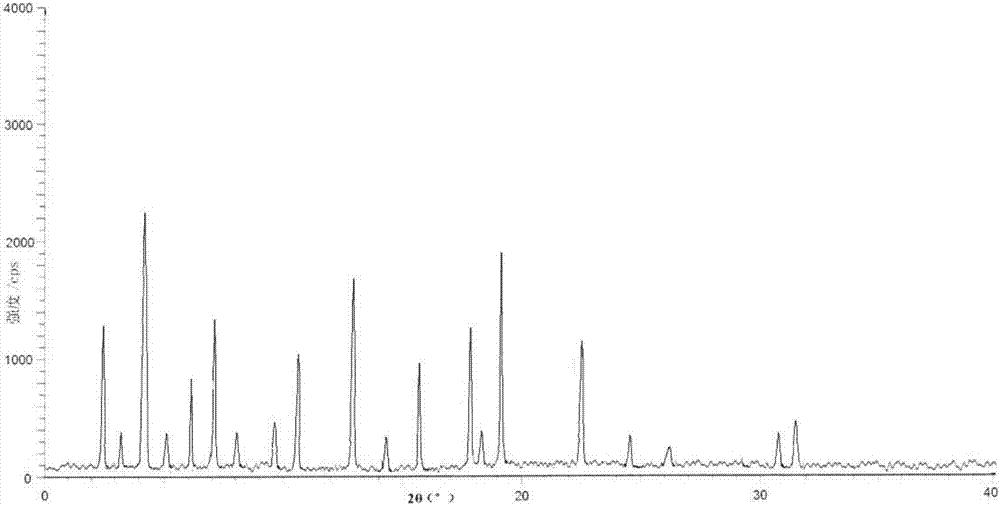
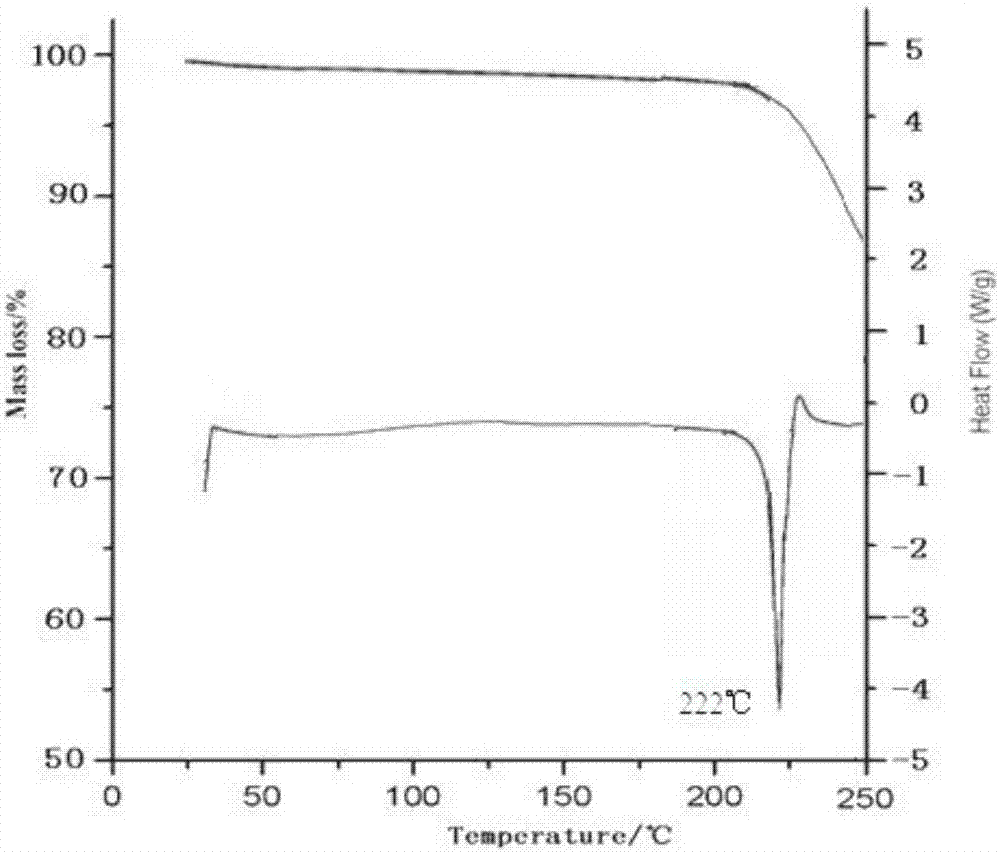
[0040] Take tofacitinib citrate 100g in the reaction flask, add 1000ml of water and ethanol mixed solution (the volume ratio of water and ethanol is 3:1), heat to 70°C, stir to dissolve, filter while hot; , while cooling down to 20°C (the cooling rate is 2°C every 10 minutes), add 2000ml of pre-cooled mixed solvent B (the volume ratio of ethanol and acetone is 1:3) to the solution at a flow rate of 1.0mL / min until crystallization , continue to cool down to -5°C (the cooling range is 1°C every 10 minutes), stir for 3h, and grow crystals for 4h. After vacuum filtration, the filter cake was vacuum-dried at 50° C. for 6 hours to obtain 90.6 g of white solid.
Embodiment 2
[0041] Embodiment 2: Preparation of tofacitinib citrate crystal compound
[0042] Take tofacitinib citrate 100g in the reaction flask, add 1500ml of water and ethanol mixed solution (the volume ratio of water and ethanol is 2:1), heat to 60°C, stir to dissolve, filter while hot; , while cooling down to 25°C (the cooling range is 2°C every 10 minutes), add 3000ml of pre-cooled mixed solvent B (the volume ratio of ethanol and acetone is 1:5) to the solution at a flow rate of 1.5mL / min until crystallization , continue to cool down to -10°C (the cooling range is 2°C every 10 minutes), stir for 2h, and grow crystals for 8h. After vacuum filtration, the filter cake was vacuum-dried at 50° C. for 4 hours to obtain 89.4 g of white solid. The X-ray powder diffraction spectrum of the obtained crystal measured by Cu-Kα rays is similar to that of Example 1.
Embodiment 3
[0043] Embodiment 3: Preparation of tofacitinib citrate crystal compound
[0044] Take 150g of tofacitinib citrate in a reaction flask, add 1500ml of a mixed solution of water and ethanol (the volume ratio of water and ethanol is 2:1), heat to 65°C, stir to dissolve, and filter while hot; , while cooling down to 30°C (the cooling range is 1°C every 10 minutes), add 3000ml of pre-cooled mixed solvent B (the volume ratio of ethanol and acetone is 1:5) to the solution at a flow rate of 2mL / min until crystallization occurs, Continue to cool down to 0°C (1°C every 10 minutes), stir for 2 hours, and grow crystals for 6 hours. After vacuum filtration, the filter cake was vacuum-dried at 50° C. for 5 hours to obtain 138 g of white solid. The X-ray powder diffraction spectrum obtained by measuring the prepared tofacitinib citrate crystals using Cu-Kα rays is similar to that of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com