New crystal form of R(+)-thioctic acid-L-lysinate and preparation method thereof
A technology of lysine salt and lipoic acid, which is applied to a new crystal form of R-lipoic acid-L-lysine salt, in the application field of medicine, which can solve the problems that the crystal form state cannot solve well, and prepare The process is cumbersome and other problems, and the effect of high dissolution rate, simple preparation process and better stability is achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
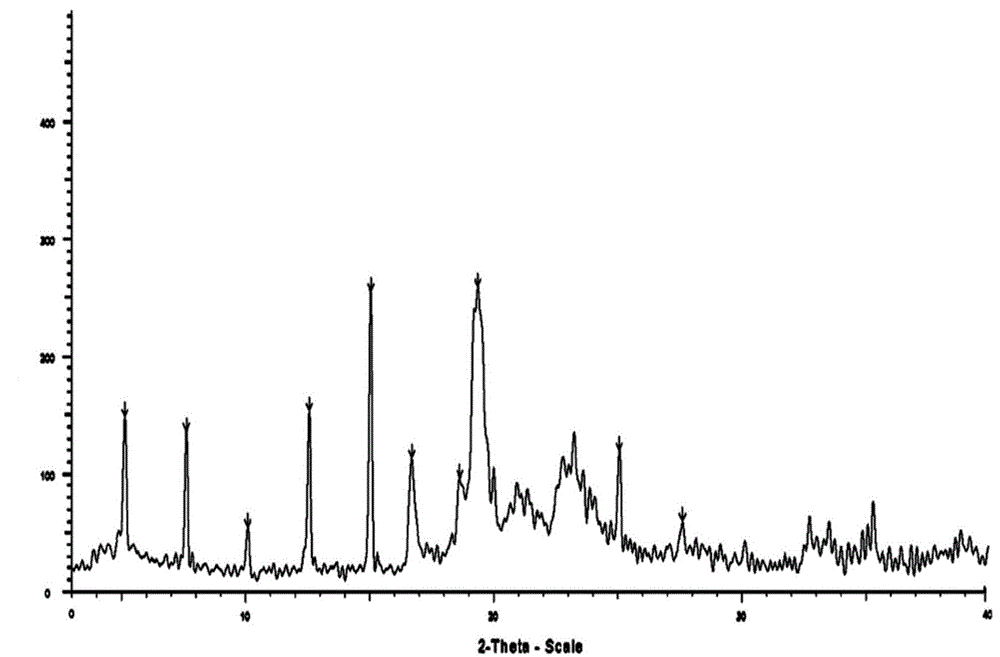
[0038] Example 1 Preparation of R(+)-lipoic acid-L-lysine salt crystal form I
[0039] At a temperature of 55-60°C, dissolve R(+)-lipoic acid (20.6g, 0.1mol) in a mixed solvent of ethanol 52mL and ethyl acetate 52mL; add dropwise L-lysine (14.6g, 0.1mol ) 40mL of 95% ethanol solution, dripping, keep warm for 2h, filter while hot; cool the filtrate to 0-10°C, stir and crystallize for 3h, filter and dry to obtain about 33.1g of yellow crystalline solid, yield 94%, HPLC 99.55% purity. The melting point is 185-186°C.
Embodiment 2
[0040] Example 2 Preparation of R(+)-lipoic acid-L-lysine salt crystal form I
[0041] At a temperature of 55-60°C, dissolve R(+)-lipoic acid (20.6g, 0.1mol) in a mixed solvent of ethanol 75mL and ethyl acetate 90mL; add dropwise L-lysine (17.5g, 0.12mol ) in 50mL of 90% ethanol solution, drop it, keep it warm for 2h, and filter it while it is hot; cool the filtrate to 0-10°C, stir and crystallize for 5h, filter and dry to obtain about 32.7g of yellow crystalline solid, with a yield of 92%, HPLC 99.60% purity. The melting point is 185-186°C.
Embodiment 3
[0042] Example 3 Preparation of R(+)-lipoic acid-L-lysine salt crystal form I
[0043] At room temperature, add sodium ethylate (15g, 0.22mol) to 120mL of ethanol solution of R(+)-lipoic acid (41.2g, 0.2mol), stir to dissolve, filter to obtain ethanol solution of R(+)-lipoic acid sodium , set aside; suspend L-lysine hydrochloride (38.4g, 0.21mol) in 150mL of acetone, control the temperature at 55-60°C, add R(+)-sodium lipoic acid solution dropwise under stirring, after the drop , keep stirring for 2 hours, and filter while hot; add 85 mL of isobutyl acetate to the filtrate, then cool down to 4°C, stir and crystallize for 3 hours, and filter and dry to obtain about 64.8 g of a yellow crystalline solid, with a yield of 92% and a purity of 99.86% by HPLC . The melting point is 187-188°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com