C-type crystal form of ceritinib, preparation method therefor and application thereof
A technology of ceritinib and crystal form, which is applied in organic chemical methods, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problems of ceritinib's poor water solubility, poor oral absorption, and limited clinical application, etc. Achieve the effect of good reproducibility, easy control and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
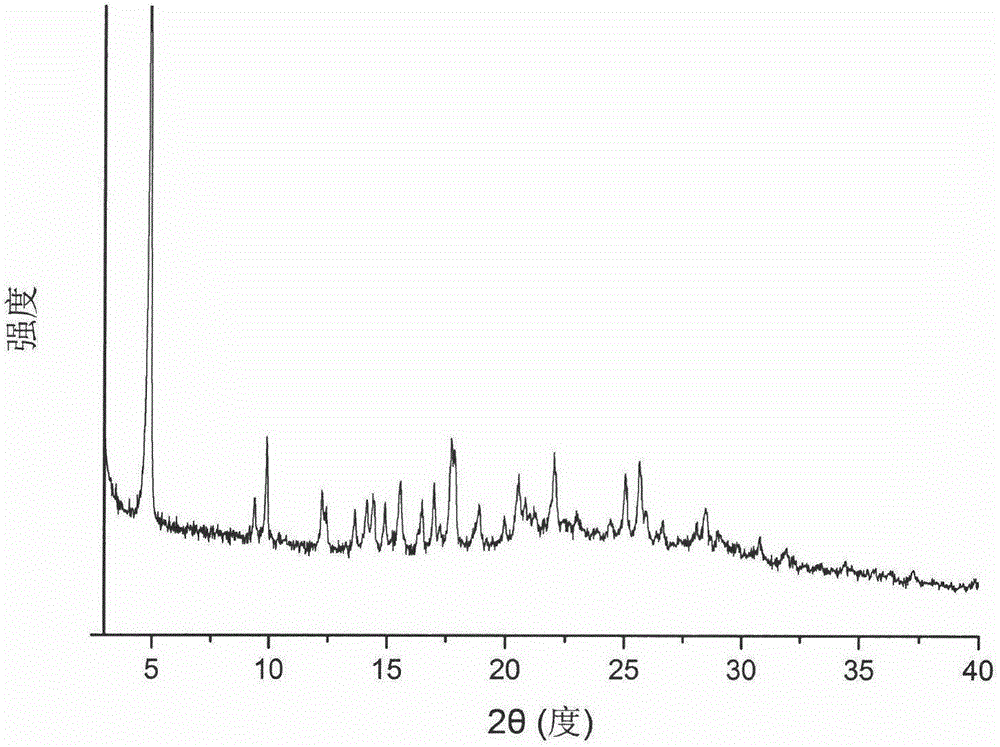
[0032] This embodiment provides a type C crystal form of Ceritinib. The X-ray powder diffraction pattern of the crystal form measured by Cu-Kα rays has 2θ angles of 4.9°, 9.4°, 9.9°, 12.2°, There are characteristic peaks at 13.7°, 14.2°, 14.4°, 14.9°, 15.6°, 16.5°, 17.0°, 17.7°, 18.9°, 20.6°, 22.0°, 25.0°, 26.6°, 25.9° and 28.5°, such as figure 1 Shown.
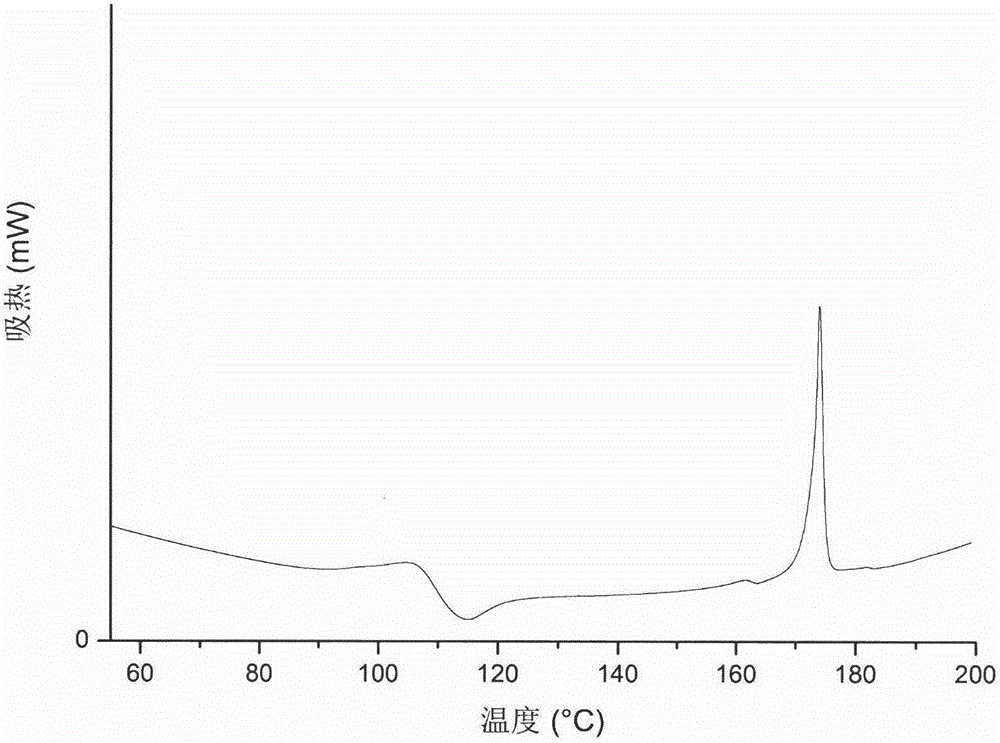
[0033] The type C crystal form of Ceritinib of this example was measured at about 100°C-120°C with a characteristic peak of endothermic and then exothermic by differential scanning calorimetry, and a characteristic melting peak was measured at about 172.9°C. Such as figure 2 Shown.
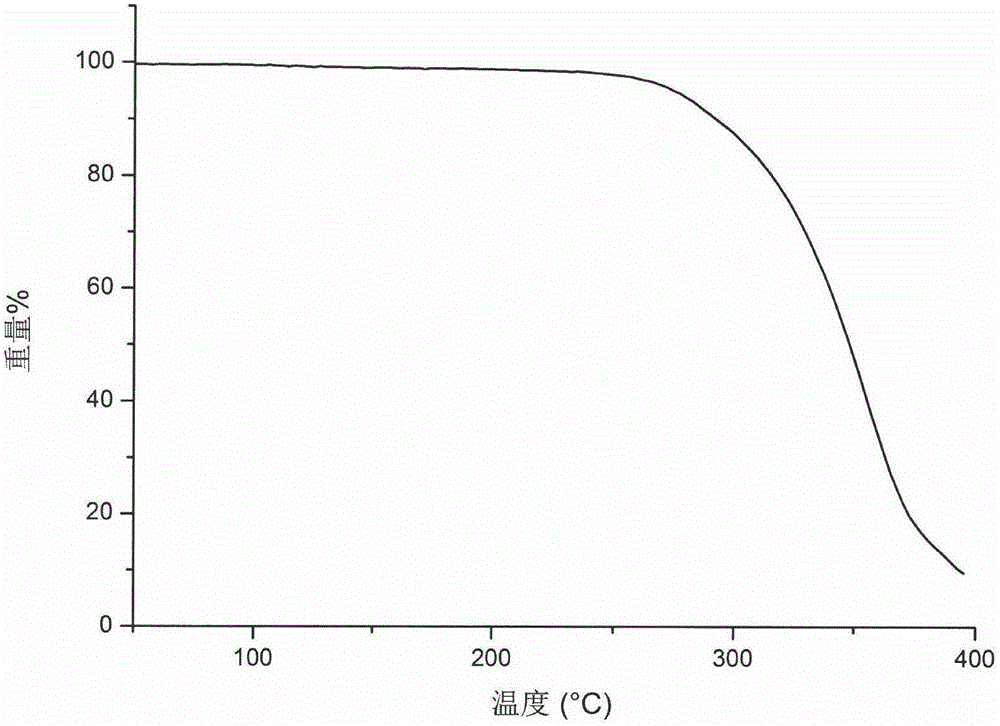
[0034] The thermogravimetric analysis diagram and infrared spectrum diagram of the C-type crystal form of Ceritinib of this embodiment are as follows image 3 with Figure 4 Shown.
[0035] The type C crystal form of Ceritinib in this example was prepared by the following method:
[0036] Place 1.0g of ceritinib in an Erlenmeyer flask, add 50 mL of met...
Embodiment 2
[0040] This example provides a type C crystal form of Ceritinib, and the qualitative determination results are the same as in Example 1.
[0041] The type C crystal form of Ceritinib in this example was prepared by the following method:
[0042] Place 1.0 g of Ceritinib in an Erlenmeyer flask, and add 50 mL of methanol. Use a magnetic stirrer to stir, and heat in a water bath at 40°C until completely dissolved to obtain a ceritinib solution; place the ceritinib solution in a refrigerator at 5°C to cool and crystallize. After the solid precipitated, it was filtered and dried under reduced pressure at room temperature. 0.95 g of white crystalline powder is obtained, which is the C crystal form of Ceritinib, and the yield is 95%.
Embodiment 3
[0044] This example provides a type C crystal form of Ceritinib, and the qualitative determination results are the same as in Example 1.
[0045] The type C crystal form of Ceritinib in this example was prepared by the following method:
[0046] Place 1.0 g of Ceritinib in an Erlenmeyer flask, and add 50 mL of methanol / acetone (volume ratio 2:1). Use a magnetic stirrer to stir, and heat in a water bath at 40°C to completely dissolve to obtain a ceritinib solution; place the ceritinib solution in a refrigerator at 5°C to cool and crystallize. After the solid precipitated, it was filtered and dried under reduced pressure at room temperature. 0.93 g of white crystalline powder is obtained, which is the type C crystal form of Ceritinib, and the yield is 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com