Highly crystalline lithium transition metal oxides
A lithium transition metal and transition metal technology, applied in the field of powdered lithium transition metal oxides, can solve the problems of low no-load voltage, increased precursor cost, poor overcharge stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: High crystallinity
[0038] a) Li 1+a m 1-a o 2±b M' k S m , where k, m=0 and M=Ni 1-c-d mn c co d .
[0039] where M=Ni 0.53 mn 0.263 co 0.2 The hydroxide MOOH was used as a precursor.
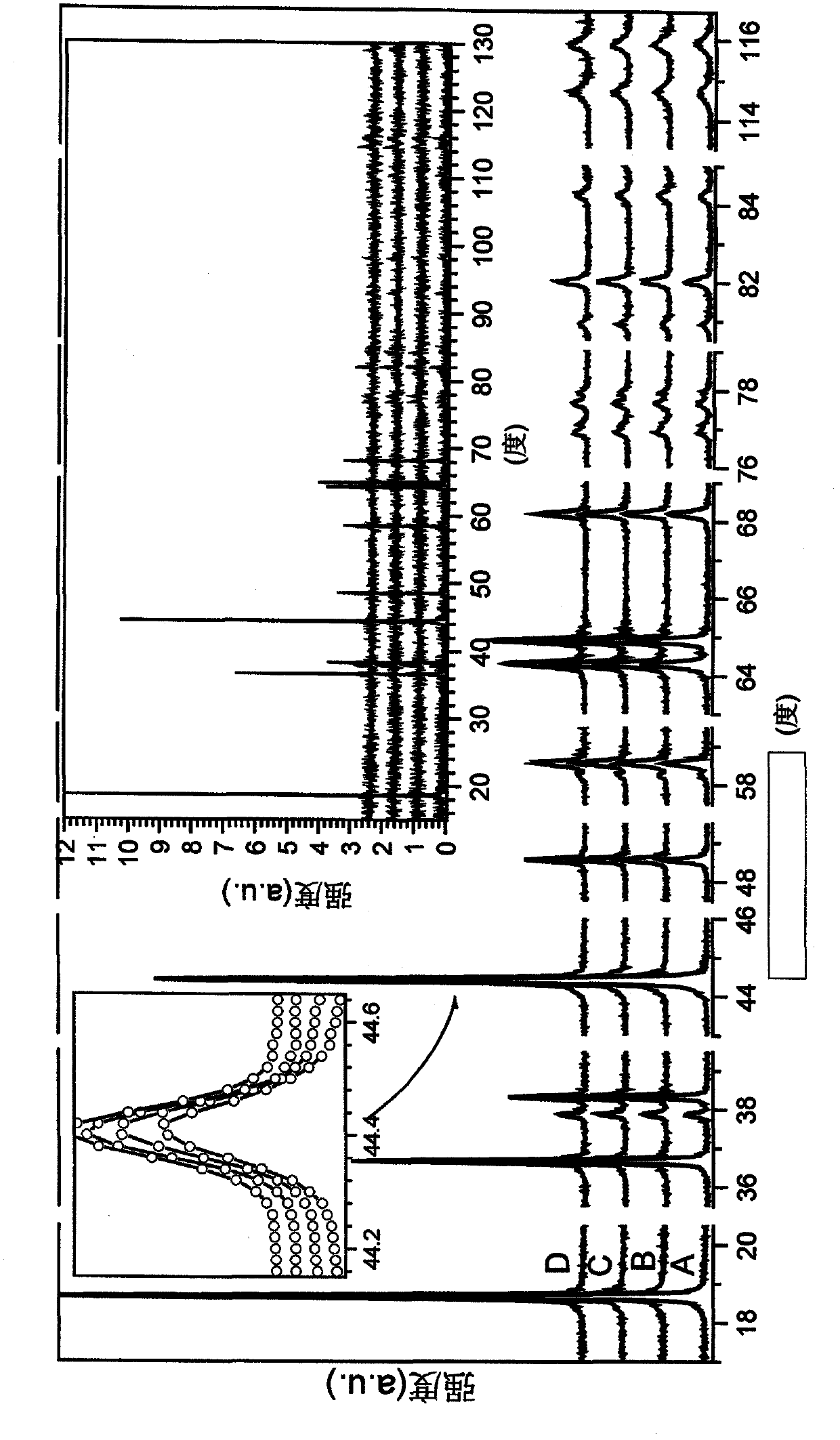
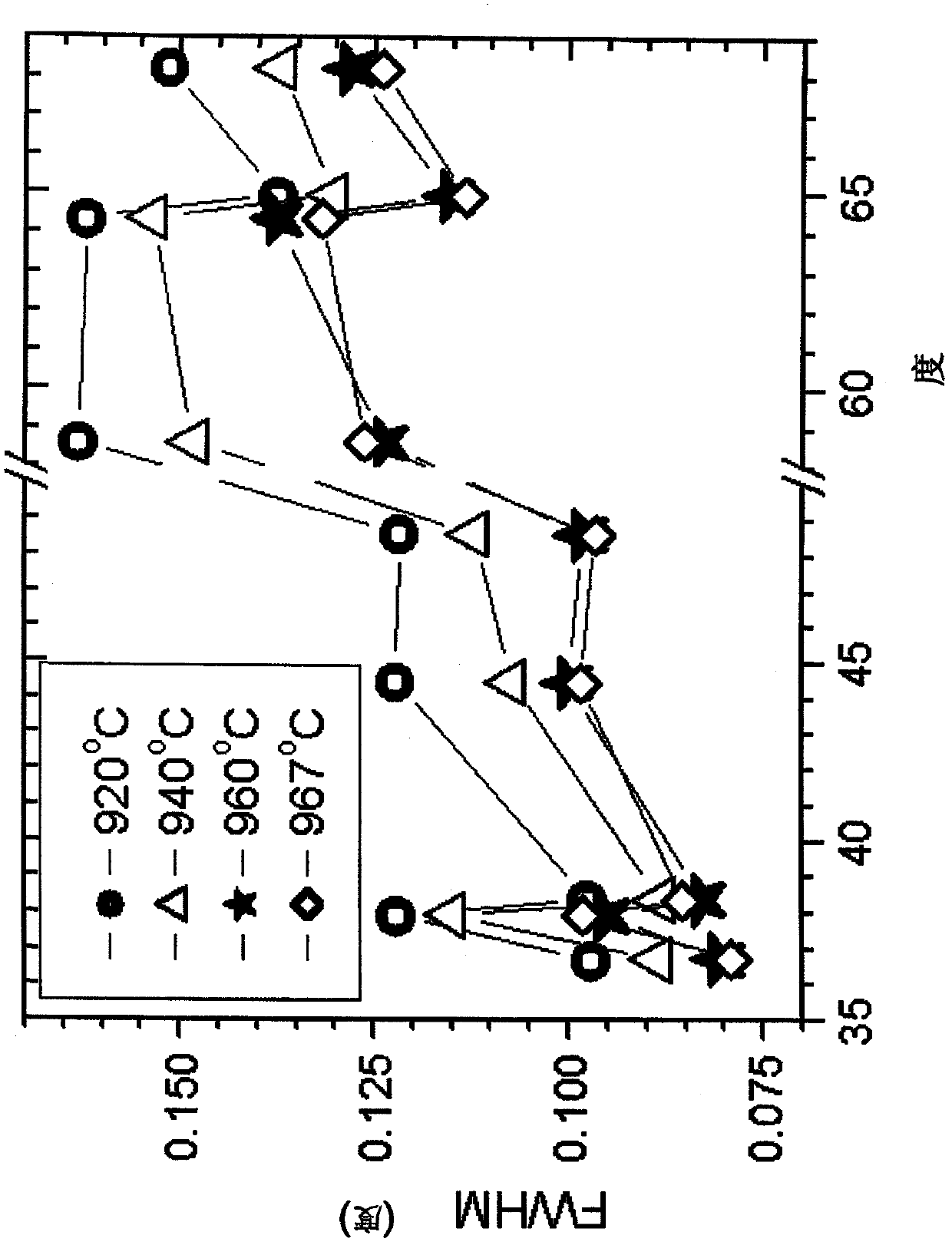
[0040] Samples were prepared at 920°C, 940°C, 960°C and at 967°C. As expected, the BET surface area decreases with increasing temperature. Li:M is essentially the same (all samples have the same unit cell volume). The electrochemical performance improves with temperature, with the best performance at a sintering temperature of approximately 960-970°C.
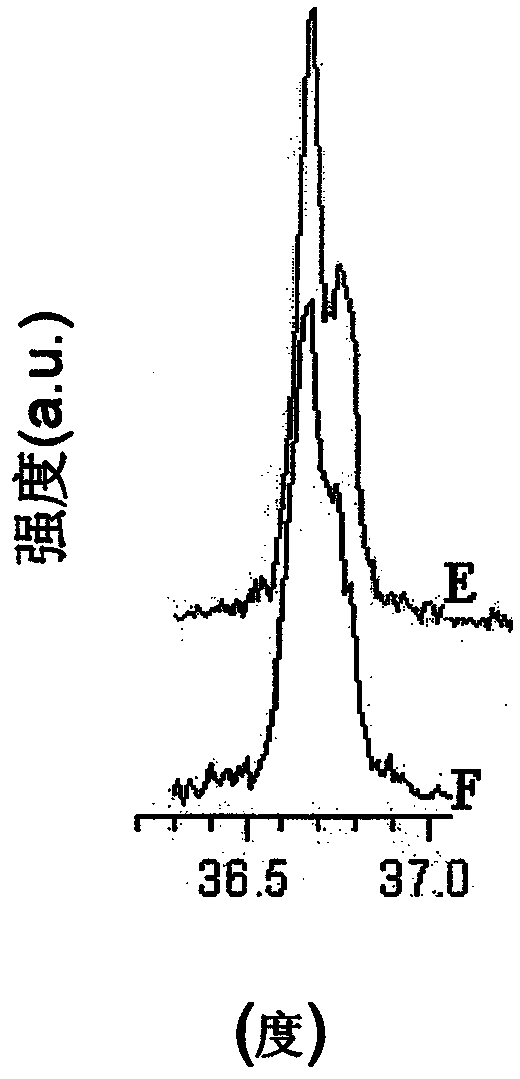
[0041] figure 1The X-ray diffraction patterns of 4 samples are shown: The sintering temperatures for samples A-D can be found in Table 1 below. The FWHM (full width at half maximum) is shown for (individual) peaks 101, 006, 102, 104, 105, 110, 108, 113 with respect to the scattering angle (degrees), the FWHM values are as described below sure as that. Peak 003 was excluded as it generally showed poorly f...
Embodiment 2
[0064] Example 2: Improved Safety and Lower Basicity of Ca-Containing Cathodes
[0065] Large-scale (several tons) synthesis of Li from mixed transition metal hydroxides 1+a m 1-a o 2 ±b Ca k S m 2 kinds of cathode materials MP1 and MP2, wherein the mixed transition metal hydroxides contain different amounts of Ca and sulfur. In both cases, the stoichiometry is very similar (a=0.05, M=Mn 1 / 3 Ni 1 / 3 co 1 / 3 , ), but the Ca levels were different: MP1 contained 393 ppm Ca, but MP2 had a typical impurity level of 120 ppm Ca (generally found above 50 but below 150 ppm). Other properties (lithium stoichiometry, particle size, BET surface area, X-ray diffraction pattern) are essentially similar.
[0066] The content of soluble base was measured as follows: 100 ml of deionized water was added to 7.5 g of the cathode, followed by stirring for 8 minutes. Typically allowed to settle for 3 minutes, then the solution was removed and passed through a 1 μm syringe filter to yiel...
Embodiment 3
[0084] Example 3: Optimization of adding Ca and sulfur
[0085] This example is used to demonstrate 2 aspects of the present invention:
[0086] (1) it confirms the observation of Example 2 that Ca "neutralizes" the negative effect of high soluble base content in sulfur-containing cathodes, and
[0087] (2) It proves that only the sulfur-containing and calcium-containing samples according to the present invention show overall good performance.
[0088] This example uses metal composition M=Mn 1 / 3 Ni 1 / 3 co 1 / 3 mixed transition metal hydroxide precursors. The precursor is generally low in Ca but contains some sulfur. Sulfur was removed after preparing primary Li-M-oxide samples (Li:M=1.1) by washing. This primary sample was then used as a precursor and made into a matrix as follows:
[0089] (6a): No addition of sulfur and calcium
[0090] (6b): Add 400ppm of Ca
[0091] (6c): Add 0.5wt% SO 4
[0092] (6d): Add 400ppm Ca and 0.5wt% SO 4 ,
[0093] Then re-sinteri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bulk density | aaaaa | aaaaa |
| Particle size distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com