Catalyst for acrolein and/or acrylic acid production and process for producing acrolein and/or acrylic acid using the catalyst
A catalyst and acrylic acid technology, which is applied in the direction of catalyst activation/preparation, preparation of organic compounds, physical/chemical process catalysts, etc. It can solve the problems of insufficient catalyst performance such as acrolein yield and service life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The reactor used in the method for producing acrolein and / or acrylic acid of the present invention is not particularly limited as long as gas-phase catalytic oxidation reaction is carried out using the catalyst of the present invention. As the reactor, any one of a fixed bed reactor, a fluid bed reactor, and a moving bed reactor can be used. The preferably used reactor is a fixed bed reactor.
[0050] As the fixed bed reactor, a tubular reactor is preferred, and a multi-tubular reactor is more preferred. In this case, the fixed-bed reactor has one or more reaction tubes, and the reaction tubes are filled with a catalyst. The reaction tubes are generally arranged in a vertical direction in a fixed bed reactor. The inner diameter of the reaction tube is not particularly limited as long as it can be filled with the catalyst, but it is usually 15 to 50 mm, more preferably 20 to 24 mm, particularly preferably 22 to 38 mm.
[0051] The catalyst filled in the reaction tube ...
Embodiment
[0055] The present invention is specifically described below by enumerating the examples, but the present invention is not subject to any limitation of the following examples, and can be implemented after making appropriate changes within the scope of the gist of the present invention, and these are all included in the technical scope of the present invention . In addition, below, for convenience, "parts by mass" may be described only as "parts".
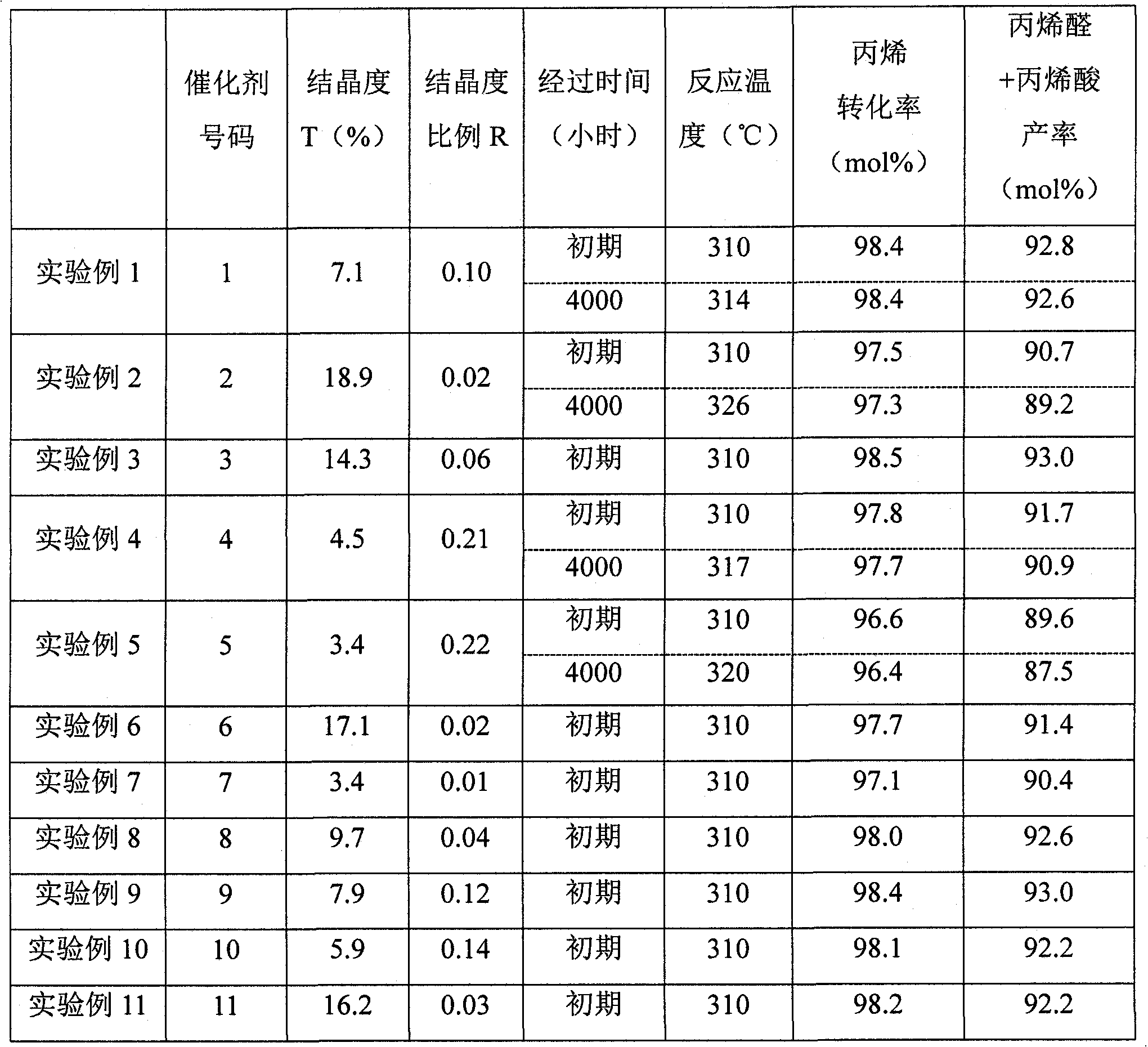
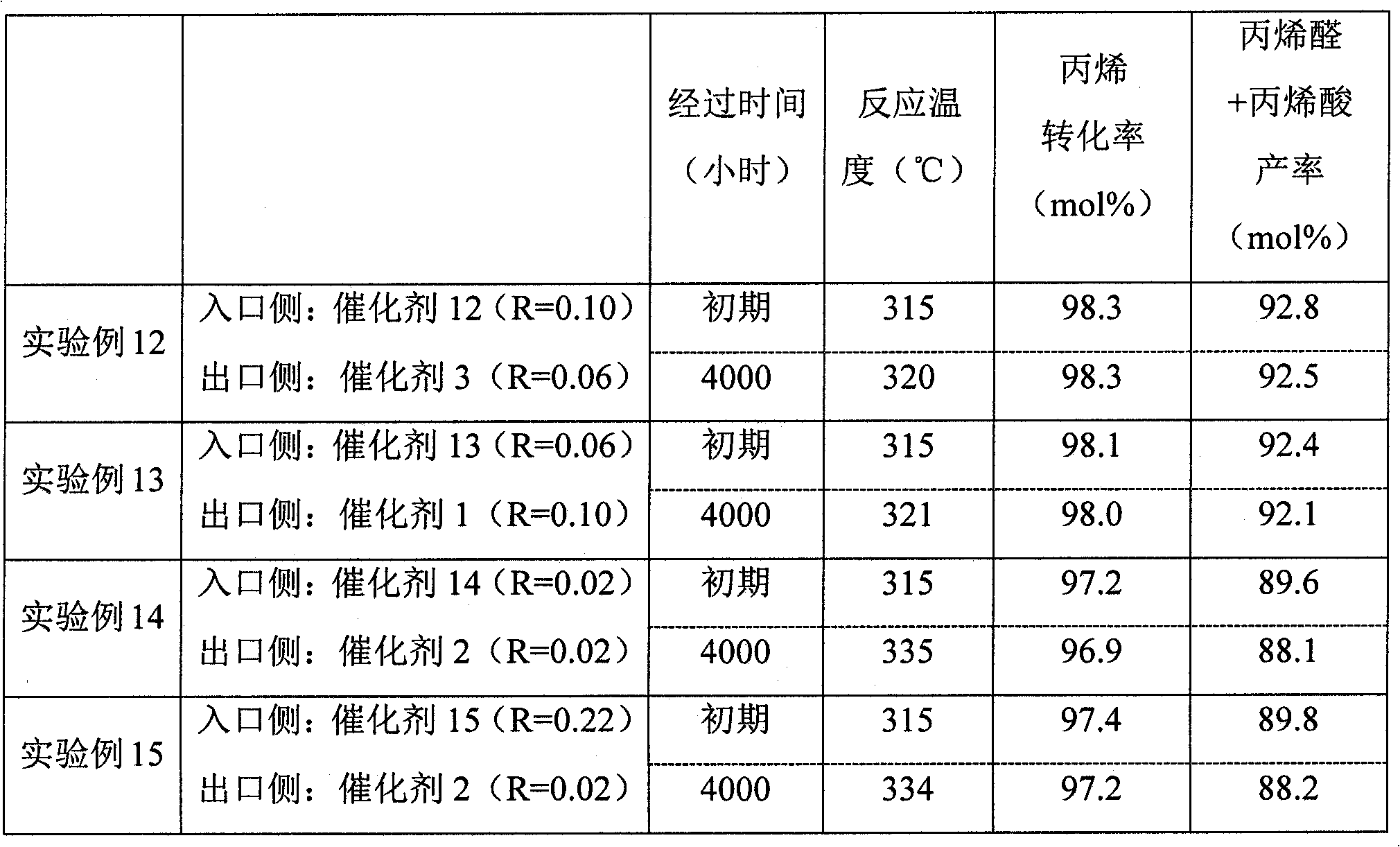
[0056] The conversion of propylene and the total yield of acrolein and acrylic acid were obtained from the following equations.
[0057] Propylene conversion (mol%)=(moles of propylene reacted) / (moles of propylene supplied)×100
[0058] Acrolein+acrylic acid yield (mol%)=(moles of acrolein and acrylic acid produced) / (moles of propylene supplied)×100
[0059] X-ray diffraction measurement was performed using X'PertPro manufactured by Spectris Corporation. The sample for X-ray diffraction measurement is prepared as follows: sieve a...
experiment example 1
[0061] (1-1) Preparation of catalyst
[0062] 3000 parts of distilled water was kept at 60° C., and 300 parts of ammonium paramolybdate and 1.7 parts of potassium nitrate were dissolved therein with stirring to prepare a liquid A. Separately, 300 parts of distilled water was kept at 60° C., 50 parts of 65% by mass nitric acid, 137 parts of bismuth nitrate, 412 parts of cobalt nitrate, 114 parts of iron nitrate, and 206 parts of nickel nitrate were added thereto under stirring to prepare liquid B. . The obtained liquid B was divided into two parts according to the mass ratio of 1:1 to obtain liquid B-1 and liquid B-2. While stirring liquid A, add liquid B-1 to liquid A over 2 minutes, and continue stirring for 30 minutes. Thereafter, liquid B-2 was also added over 2 minutes, and stirring was continued for 30 minutes to obtain a suspension. 200 parts of ammonium paramolybdate was added again to the obtained suspension, and stirring was continued for 30 minutes to obtain a sta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com