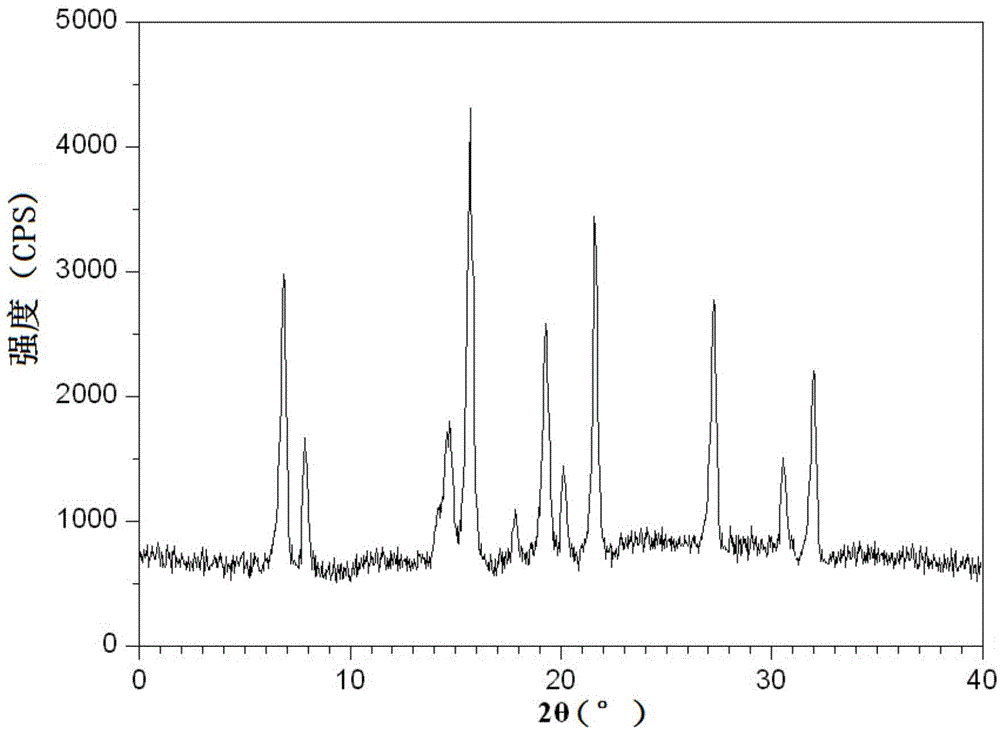
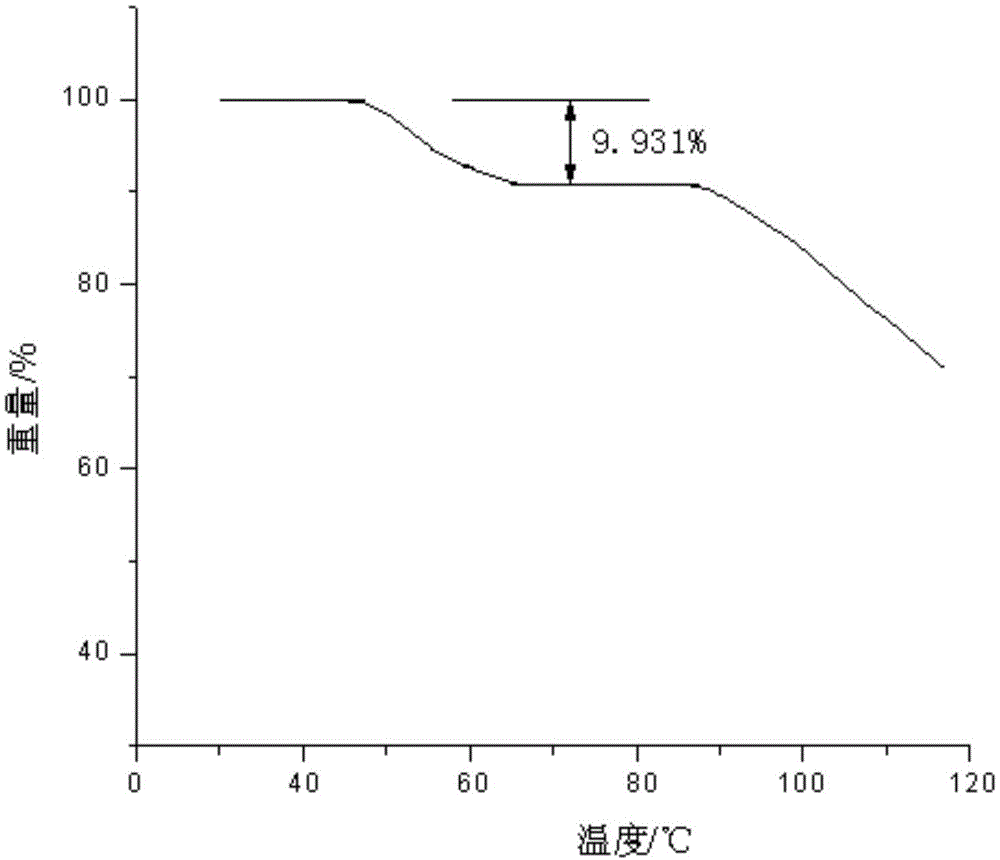
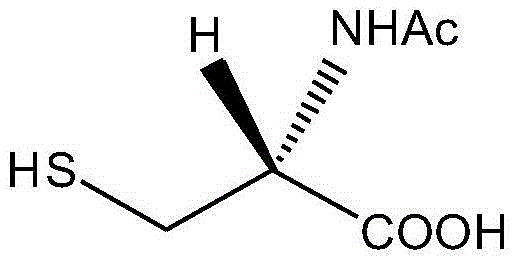
Acetylcysteine compound and acetylcysteine solution being used for inhalation and containing same
A technology of acetylcysteine and its compound, which is applied in the field of acetylcysteine solution for inhalation, can solve problems such as the difference in solubility of crystalline drugs, large free energy per unit surface, and easy hydration of the surface between particles, so as to improve the inhalation Moist, quick onset, easy to use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] [Example 1] Preparation of acetylcysteine compound
[0041] 1) Dissolve 12kg of acetylcysteine in 10L of water to obtain a 1.2kg / L aqueous solution of acetylcysteine;
[0042] 2) Add 0.01% (w / v) activated carbon to the acetylcysteine aqueous solution obtained in step 1), stir, suction filter, and take the filtrate;
[0043] 3) Add 50L of mixed solvent A to the filtrate obtained in step 2) with a flow rate of 13L / min under stirring at a speed of 50r / min to form a turbid solution, wherein said mixed solvent A is tetrahydrofuran and n-butanol by volume A mixed solvent with a ratio of 2:1;
[0044] 4) Place the turbid solution obtained in step 3) at a frequency of 3.5kHz and an intensity of 0.6W·cm -2 Under the ultrasonic field of 6L / min, 120L of mixed solvent B was added to it at a flow rate of 6L / min. After the addition, crystals were precipitated, wherein said mixed solvent B was a mixed solvent composed of diethyl ether and isopropyl ether at a ratio of 5:1;
...
Embodiment 2
[0048] [Example 2] Preparation of acetylcysteine compound
[0049] 1) Dissolve 26kg of acetylcysteine in 10L of water to obtain a 2.6kg / L aqueous solution of acetylcysteine;
[0050] 2) Add 0.01% (w / v) activated carbon to the acetylcysteine aqueous solution obtained in step 1), stir, suction filter, and take the filtrate;
[0051] 3) Add 100L of mixed solvent A to the filtrate obtained in step 2) at a flow rate of 20L / min under stirring at a speed of 60r / min to form a turbid solution, wherein said mixed solvent A is tetrahydrofuran and n-butanol by volume A mixed solvent with a ratio of 5:1;
[0052] 4) Place the turbid solution obtained in step 3) at a frequency of 6.5kHz and an intensity of 4W·cm -2 Under the ultrasonic field of 12L / min, 180L of mixed solvent B was added to it at a flow rate of 12L / min. After the addition, crystals were precipitated, wherein said mixed solvent B was a mixed solvent composed of diethyl ether and isopropyl ether at a ratio of 7:1;
[...
Embodiment 3
[0055] [Example 3] Preparation of acetylcysteine compound
[0056] 1) Dissolve 20kg of acetylcysteine in 10L of water to obtain a 2.0kg / L aqueous solution of acetylcysteine;
[0057] 2) Add 0.01% (w / v) activated carbon to the acetylcysteine aqueous solution obtained in step 1), stir, suction filter, and take the filtrate;
[0058] 3) Add 80L of mixed solvent A at a flow rate of 16L / min to the filtrate obtained in step 2) under stirring at a speed of 55r / min to form a turbid solution, wherein said mixed solvent A is tetrahydrofuran and n-butanol by volume A mixed solvent with a ratio of 4:1;
[0059] 4) Place the turbid solution obtained in step 3) at a frequency of 4.5kHz and an intensity of 2W·cm -2 Under the ultrasonic field of 8L / min, 150L of mixed solvent B was added to it at a flow rate of 8L / min. After the addition, crystals were precipitated, wherein said mixed solvent B was a mixed solvent composed of diethyl ether and isopropyl ether at a ratio of 6:1;
[006...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Outer diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com