Methylpyrazine derivative biological arginine hydrate
A technology of methylpyrazine and its derivatives, which is applied in the field of methylpyrazine derivative arginine hydrate, can solve the problem of acipimus crystallographic characterization parameters that are not mentioned, less crystal eutectic structure, and production problems. Low rate and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
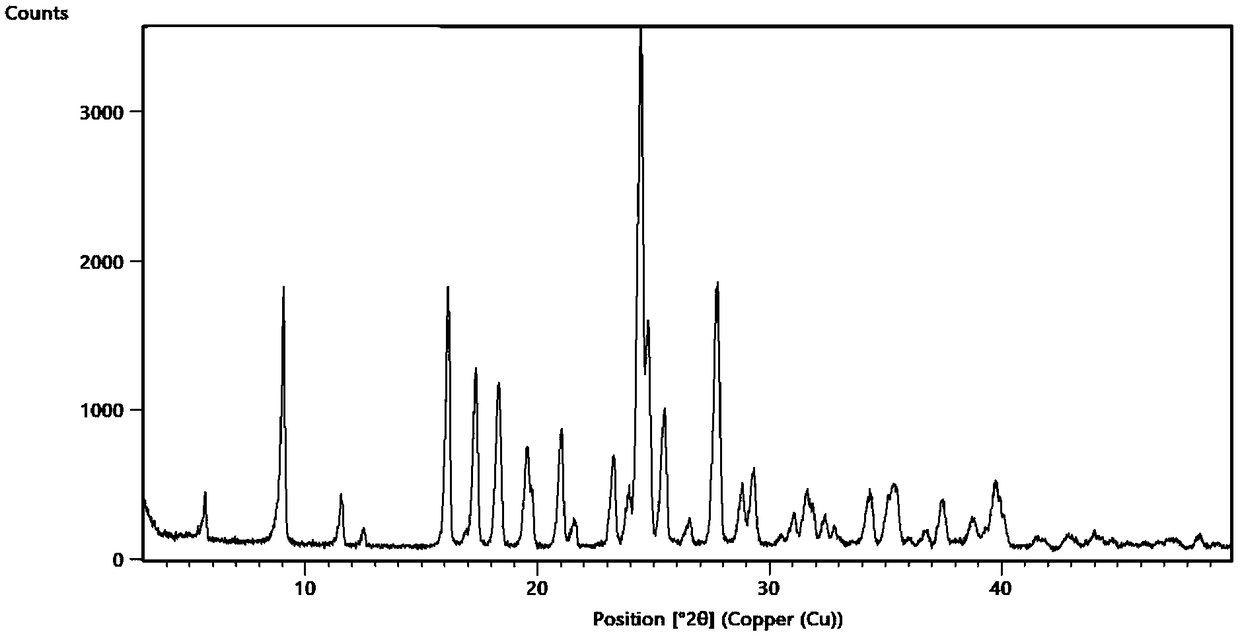
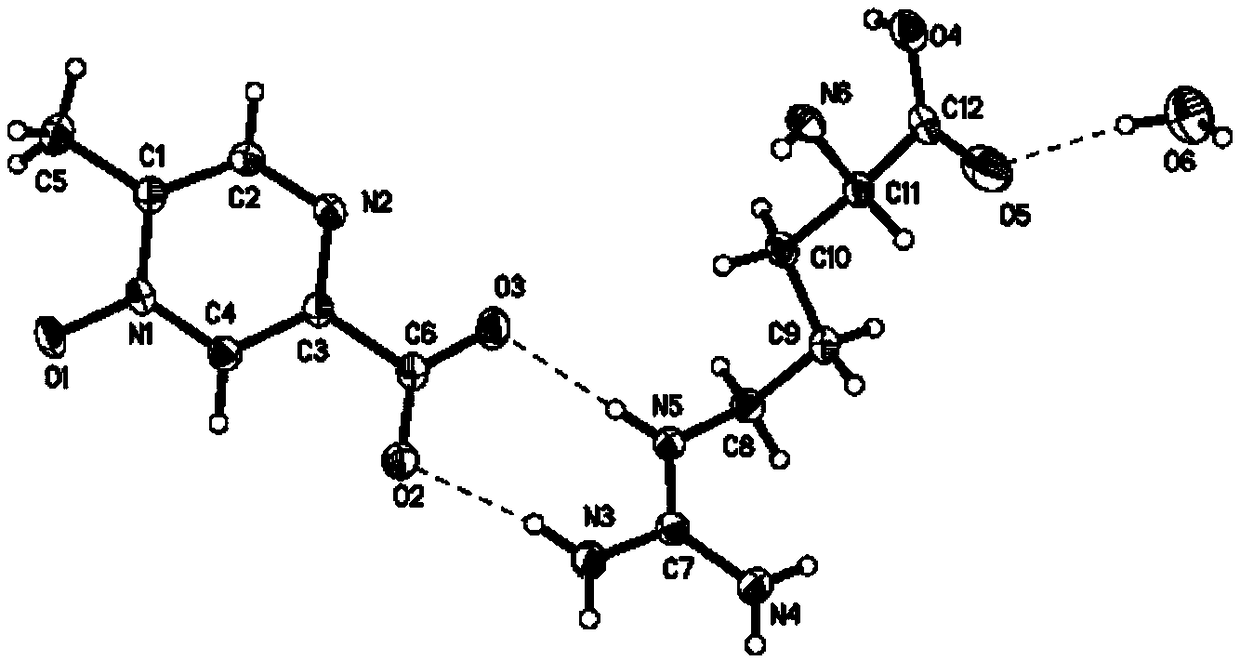
[0067] Dissolve 5.0g (32.4mmol) of methylpyrazine derivatives and 5.6g (32.4mmol) of arginine in aqueous methanol (50ml of methanol + 0.5ml of water) and heat to 60°C to dissolve. After the solution is clear, cool down to 20 ℃, static crystallization for 52 hours, filtered and dried to obtain 10.9 g of arginine hydrate, a methylpyrazine derivative, with a yield of 97.1%, HPLC: 99.94%, and impurity I: 0.05%.
Embodiment 2
[0069] Dissolve 5.0g (32.4mmol) of methylpyrazine derivatives and 11.2g (48.6mmol) of arginine in 50ml of ethyl acetate aqueous solution (50ml of ethyl acetate + 2ml of water), heat to 75°C to dissolve, and the solution is clarified , cooled to 15° C., static crystallization for 48 hours, filtered and dried to obtain 11.0 g of arginine hydrate, a methylpyrazine derivative, with a yield of 98.0%, HPLC: 99.93%, and impurity I: 0.06%.
Embodiment 3
[0071] Dissolve 10.0g (64.8mmol) of methylpyrazine derivatives and 13.4g (77.8mmol) of arginine amine in aqueous tetrahydrofuran (50ml of tetrahydrofuran + 0.1ml of water), and heat to 65°C to dissolve. After the solution is clear, cool down to Static crystallization at 10°C for 36 hours, filtration and drying to obtain 21.6 g of arginine hydrate, a methylpyrazine derivative, with a yield of 96.3%, HPLC: 99.92%, and impurity I: 0.07%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com