Novel polymorph of safinamide and preparation method therefor
A technology of safinamide mesylate and crystal form, applied in the field of medicinal chemistry, can solve the problems of low dissolution rate, poor stability and the like, and achieve the effects of high dissolution rate, high stability and favorable industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
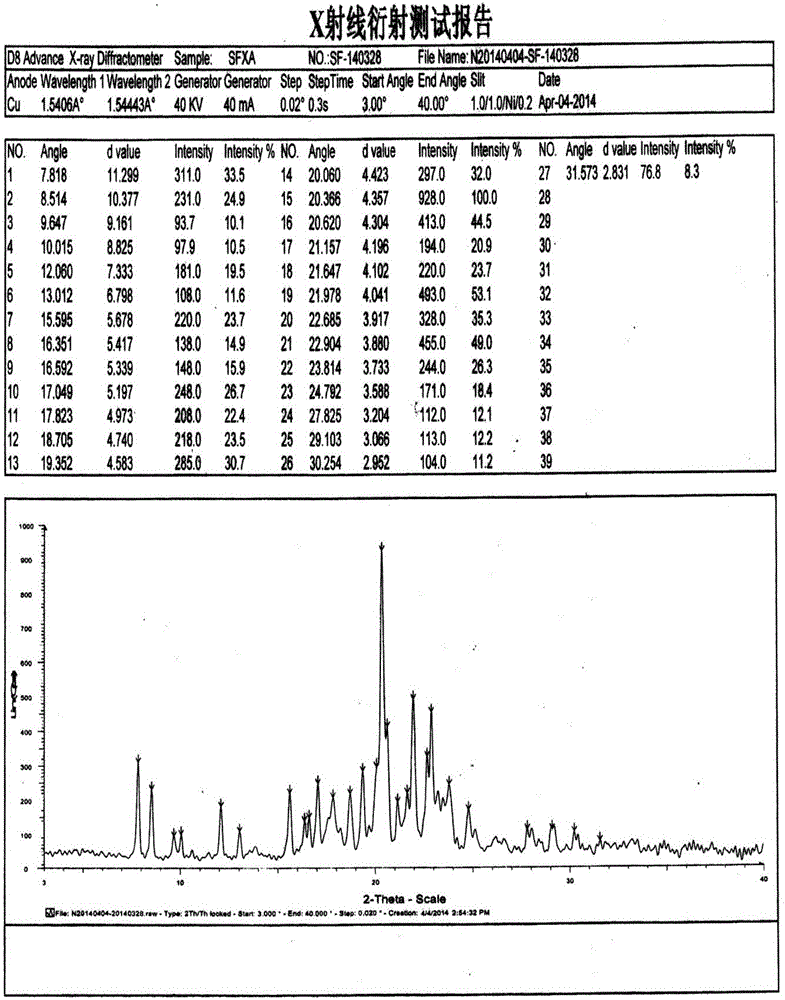
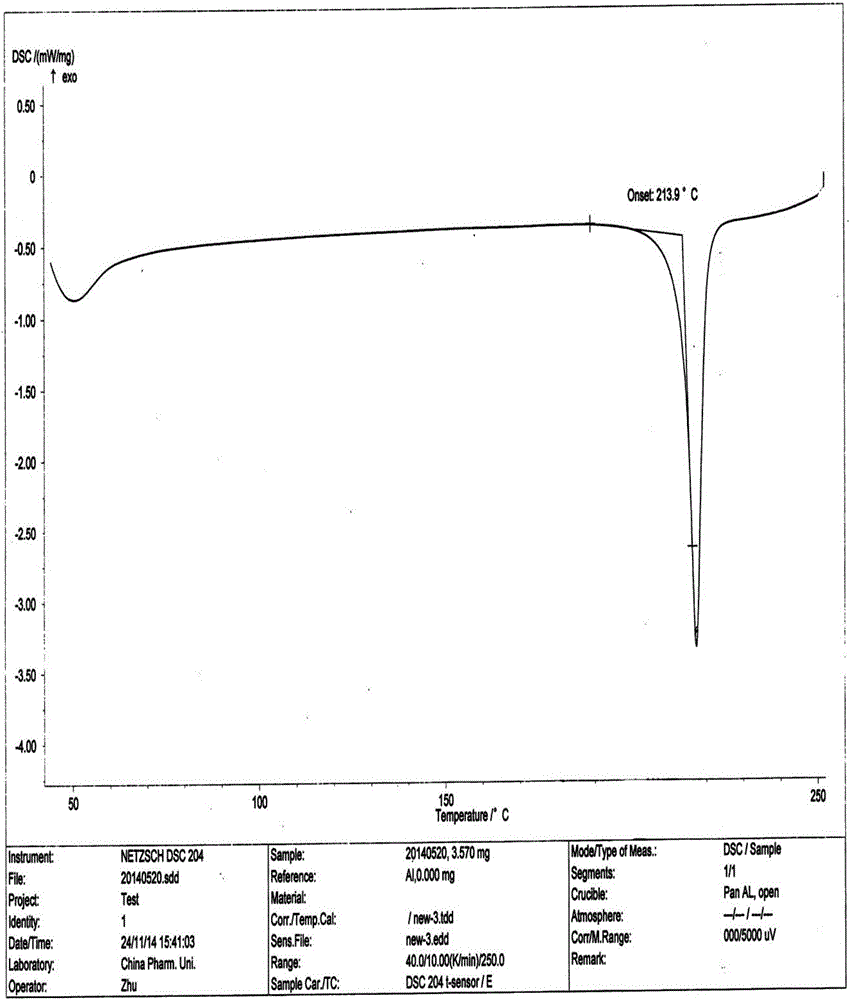
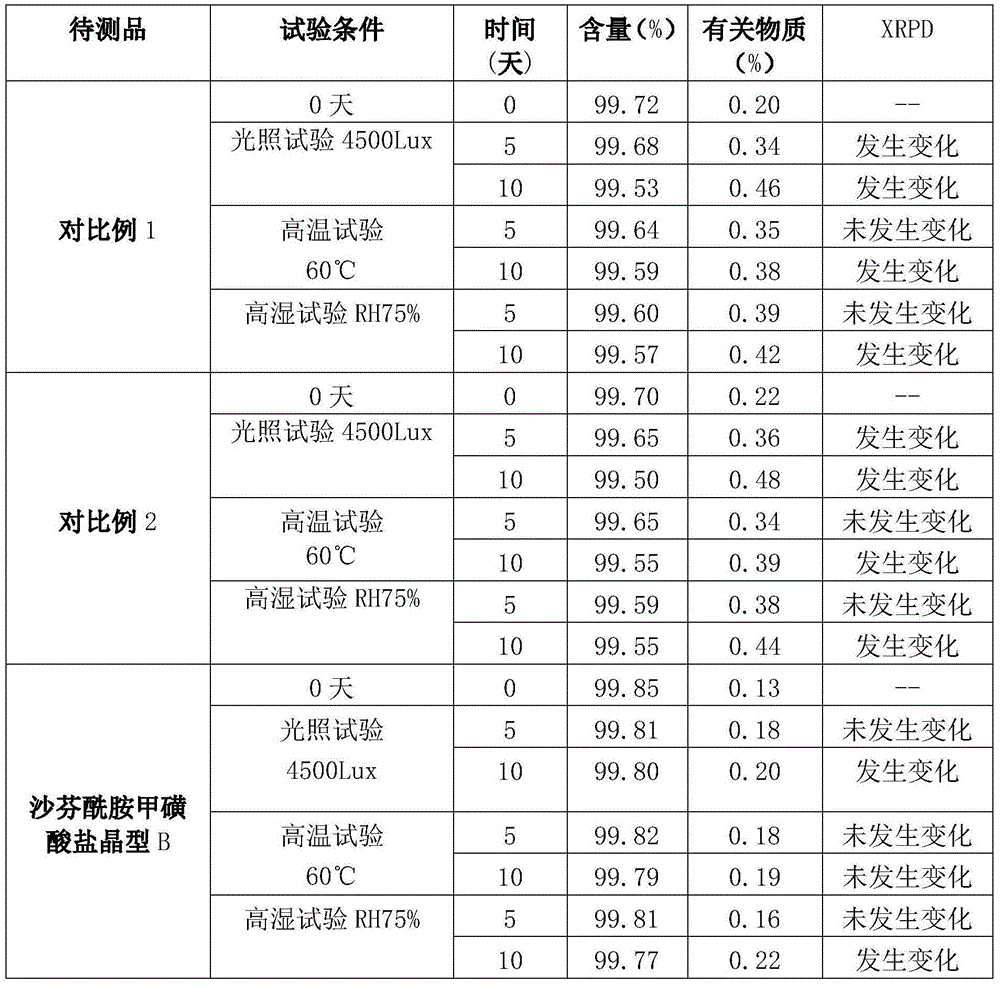
[0036] The preparation of embodiment 1 safinamide methanesulfonate crystal form B
[0037] At a temperature of 45-50°C, stir and dissolve 10 g of safinamide mesylate in a mixed solvent of ethanol and water, wherein the volume of ethanol is 6 times the weight of safinamide mesylate, that is, 60 mL; The volume is 1 times the weight of safinamide methanesulfonate, that is, 10mL. After dissolving, filter while it is hot, cool the filtrate to 20-25°C, slowly stir and crystallize, filter the precipitated crystals, and dry under vacuum at 35-40°C to obtain 9.6 g, yield 96%.
Embodiment 2
[0038] The preparation of embodiment 2 safinamide methanesulfonate crystal form B
[0039]At a temperature of 45-50°C, stir and dissolve 10 g of safinamide mesylate in a mixed solvent of methanol and water, wherein the volume of methanol is 5 times the weight of safinamide mesylate, that is, 50 mL; The volume is 0.8 times the weight of safinamide methanesulfonate, that is, 8mL. After dissolving, filter while it is hot, cool the filtrate to 20-25°C, slowly stir and crystallize, filter the precipitated crystals, and vacuum-dry at 35-40°C to obtain 9.4 g, yield 94%.
Embodiment 3
[0040] The preparation of embodiment 3 safinamide methanesulfonate crystal form B
[0041] At a temperature of 45-50°C, 10 g of safinamide mesylate was stirred and dissolved in a mixed solvent of isopropanol and water, wherein the volume of isopropanol was 7 times the weight of safinamide mesylate, i.e. 70mL; the volume of water is 1.2 times the weight of safinamide methanesulfonate, that is, 12mL. After dissolving, filter while hot, cool the filtrate to 20-25°C, stir slowly to crystallize, filter the precipitated crystals, and vacuum dry at 35-40°C. That is, 9.5 g was obtained, and the yield was 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com