Gastrodin compound and preparation thereof
A technology for gastrodin and compound, applied in the field of gastrodin compounds and preparations thereof, can solve the problems of poor storage stability of gastrodin, loss of active ingredients, and reduced curative effect, etc., achieve good comprehensive physical and chemical properties, improve drug safety, and storage stability Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of Gastrodin Compounds
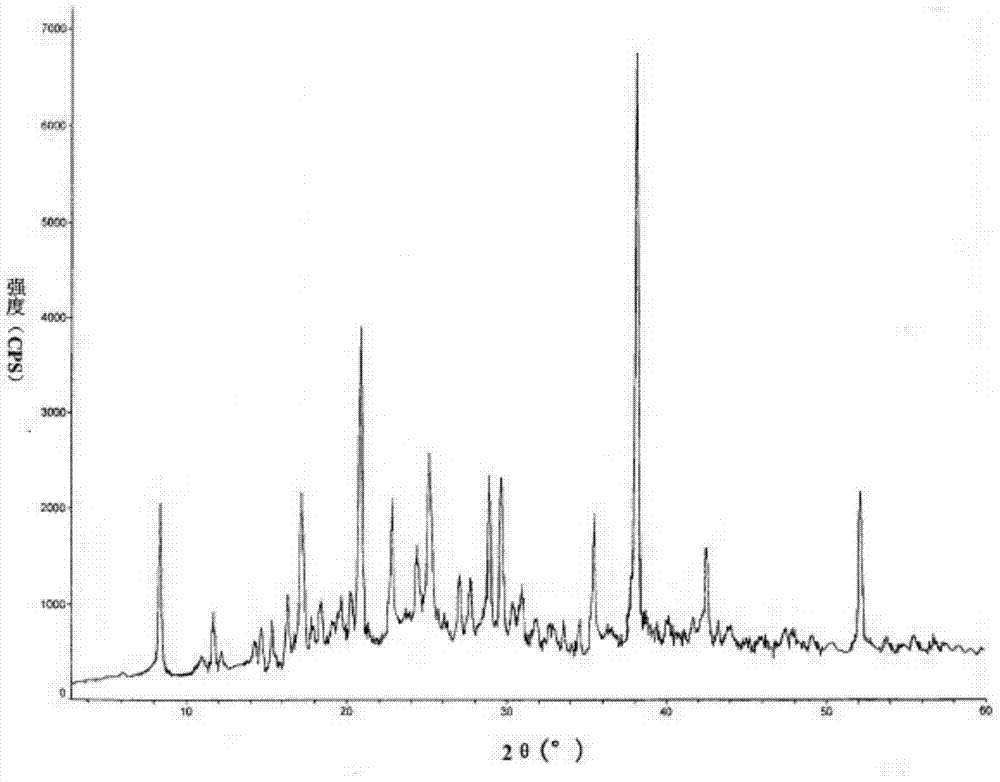
[0038] Take 50g of gastrodin crude product, add acetone to make a saturated solution at 40°C, stir for 40min, adjust the pH to 7, then add dimethyl sulfoxide whose volume is 1 / 5 of the volume of acetone, continue to heat up to 55°C and keep stirring for 0.5h, add For activated carbon decolorization, the amount of activated carbon added is 0.3% g / ml of the total volume of the liquid medicine, stirred and adsorbed for 30 minutes, decarbonized and sterilized by filtration; chloroform at 45°C is slowly added at a stirring rate of 30 rpm, and the volume of chloroform used is 1 / 2 of the volume of acetone. 4 times, after the addition, continue to stir at a rate of 20rmp and cool down to room temperature naturally, wash with acetone for 3 times, and dry under reduced pressure for 2h to obtain the final product. Yield 64.8%, HPLC content 99.90%.
[0039] The X-ray powder diffraction spectrum obtained by Cu-Kα ray measurement is figure 1 dis...
Embodiment 2
[0041] Preparation of Gastrodin Compounds
[0042]Take 50g of crude gastrodin, add acetone to make a saturated solution at 30°C, stir for 20min, adjust the pH to 6, then add dimethyl sulfoxide whose volume is 1 / 7 of the volume of acetone, continue to heat up to 45°C and keep stirring for 3h, add active For carbon decolorization, the amount of activated carbon added is 0.3% g / ml of the total volume of the liquid medicine, stirred and adsorbed for 30 minutes, decarbonized and sterilized by filtration; 55°C chloroform is slowly added at a stirring rate of 20 rpm, and the volume of chloroform used is 8% of the volume of acetone. After the addition, continue to stir at a rate of 10rmp and cool down to room temperature naturally, wash with acetone for 3 times, and dry under reduced pressure for 2h to obtain the product. The yield is 66.9%, and the HPLC content is 99.92%.
[0043] The X-ray powder diffraction pattern obtained by Cu-Kα ray measurement is consistent with the result of...
Embodiment 3
[0045] Preparation of Gastrodin Injection
[0046]
[0047] The ampoules are cleaned as usual for injections, first the initial washing and then the fine washing (the water for injection in the fine washing needs to be filtered through a 0.45 μm microporous membrane), then dried and sterilized for later use. Weigh gastrodin according to the prescribed amount, put it in an appropriate sterile container, add an appropriate amount of water for injection, stir to dissolve it, add water for injection until the prescribed amount is constant, and mix well. Add 0.05% (g / ml) activated carbon for needles, stir and adsorb at room temperature for 20 minutes, and filter to decarbonize. Filter with a 0.22 μm microporous membrane. Sampling, intermediate inspection (pH value, content, clarity), subpackage into 1ml ampoules after passing the test, make 1000 bottles, and melt seal. Autoclave at 115°C for 30 minutes. Light inspection, packaging, inspection, storage.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com