Agomelatine and medicine composition thereof
A technology of agomelatine and composition, applied in the field of medicinal chemical synthesis, can solve problems such as weight change, sexual dysfunction, drug withdrawal syndrome, etc., and achieve the effects of facilitating absorption, good solubility and high quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
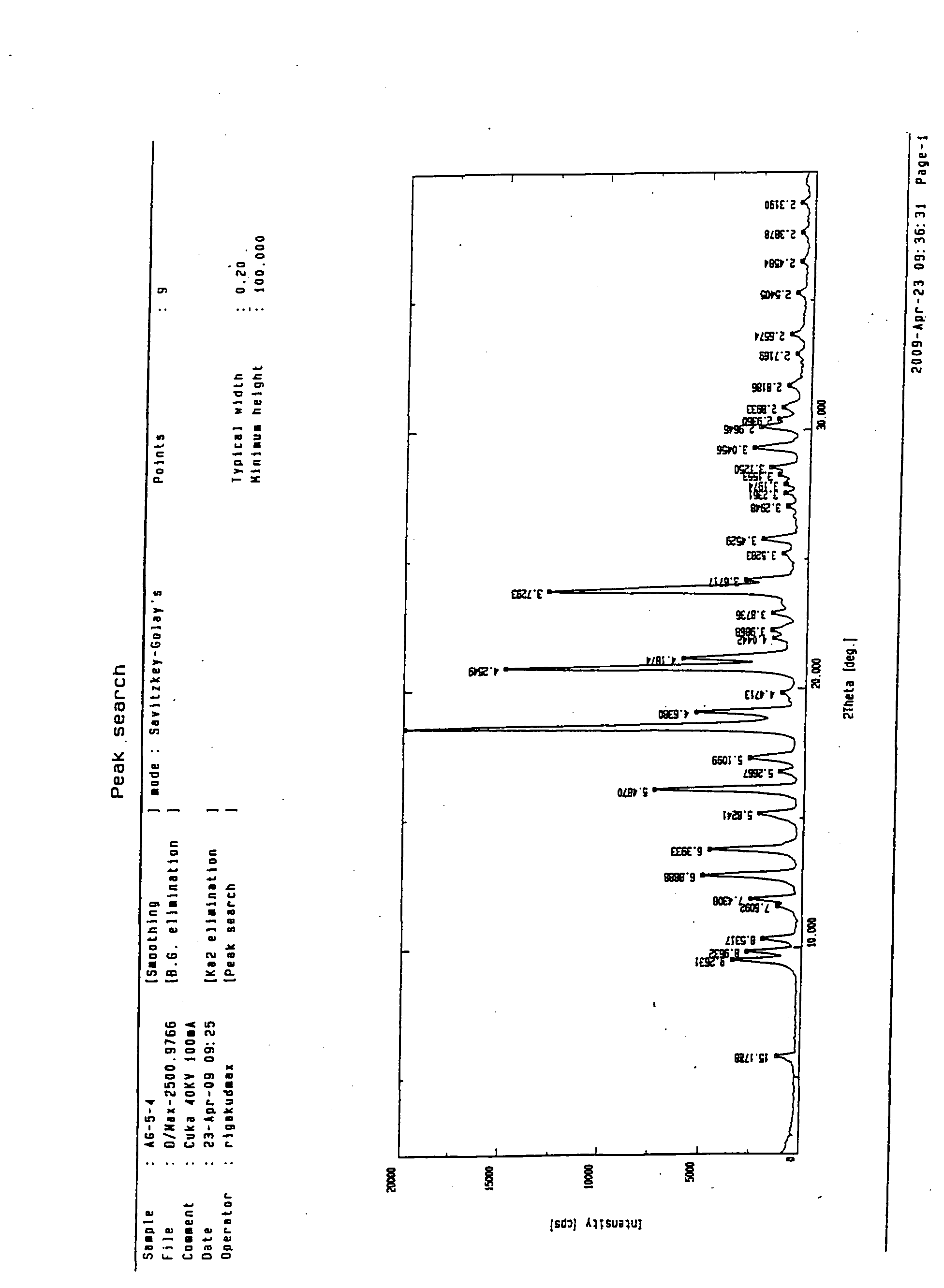
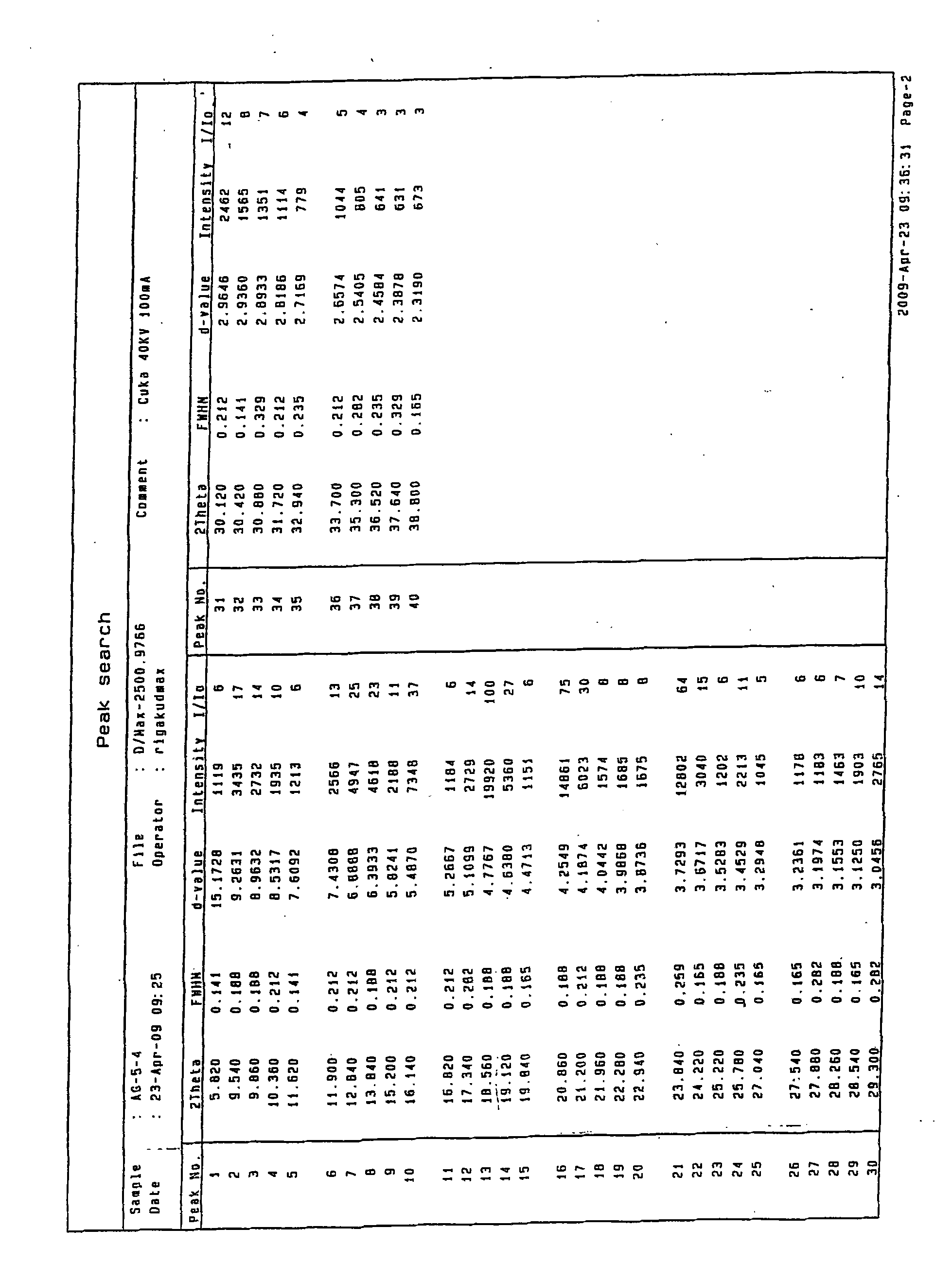
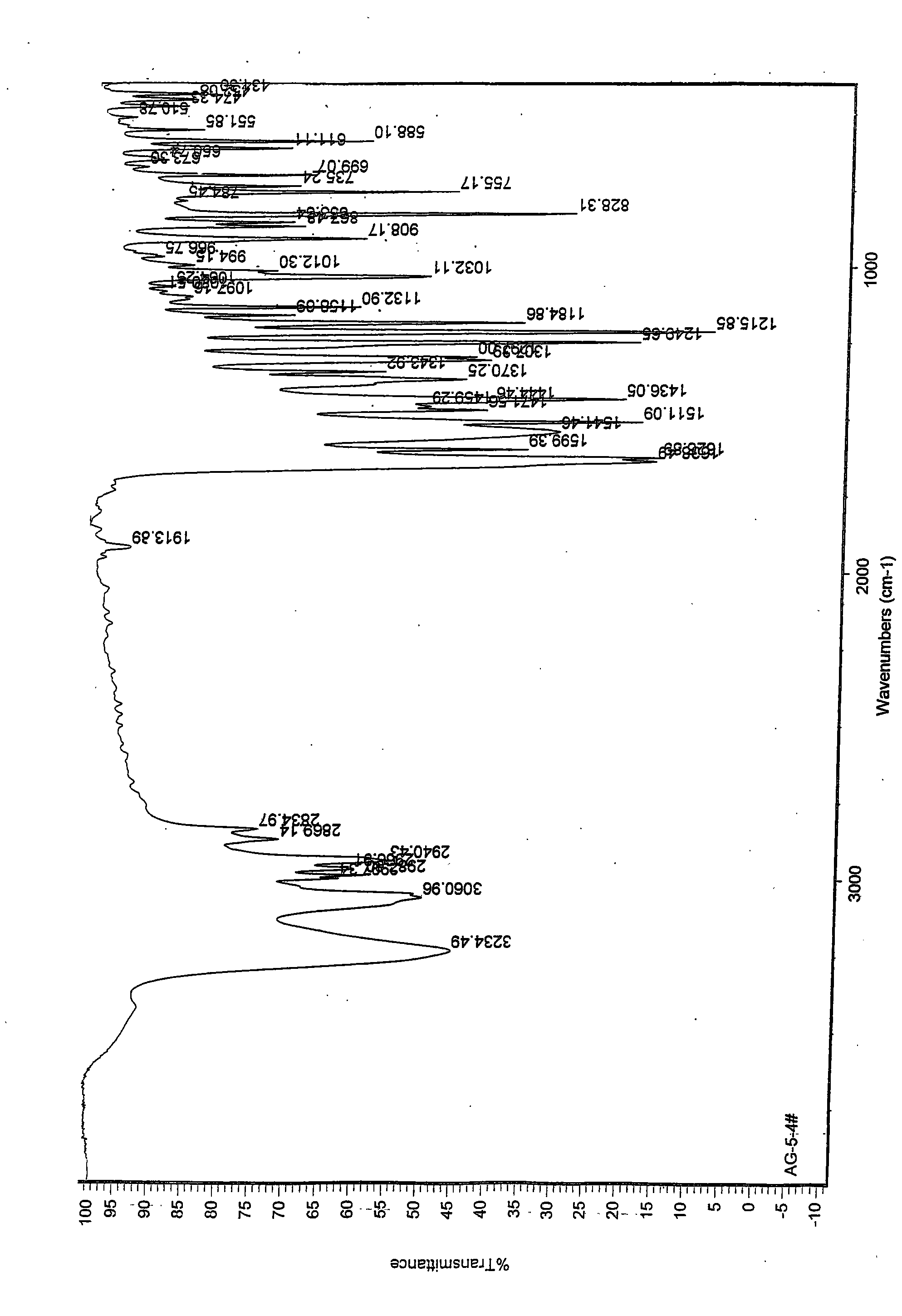
Image
Examples
Embodiment 2
[0058] Preparation of (7-methoxy-1-naphthyl)-ethyl acetate
[0059] Add 17.6g elemental sulfur and 90g (7-methoxy-1,2,3,4-tetrahydro-1-naphthenyl)-ethyl acetate to the reaction flask, stir and heat to 215°C, and react for 10 hours . After the reaction was completed, it was naturally cooled to 60°C, 500ml of ethyl acetate was added, and the mixture was stirred for 30min. Filter, wash the filter cake with 100 ml of ethyl acetate, combine the filtrates, and evaporate to dryness under reduced pressure to obtain 92 g of brown oil with a content of 82.4% (HPLC) and a pure yield of 85%.
Embodiment 3
[0061] Preparation of 7-methoxy-1-naphthylacetic acid
[0062] Dissolve 40g of sodium hydroxide in 1000ml of water, add 1000ml of 95% ethanol, and mix well. Then 50 g of (7-methoxy-1-naphthyl)-ethyl acetate was added to the above mixed solution, and stirred at room temperature for 3 hours. The reaction was stopped, and the ethanol was evaporated under reduced pressure to obtain a brown-red liquid. After washing with 300ml of ethyl acetate × 2, 30ml of 95% ethanol was added to the water layer, and under rapid stirring, concentrated hydrochloric acid was added dropwise to adjust the pH to 2, and a large amount of shallow brown solid. Filtrate and dry to obtain 32g of the product, mp154-156°C, the content determined by HPLC is 98.48%, and the yield is 72%.
[0063] Reference Example 4:
[0064] Preparation of 7-methoxy-1-naphthylacetamide
[0065] Add 50g of 7-methoxy-1-naphthyl acetic acid into 750ml of dichloromethane, heat to dissolve, and slowly add thionyl chloride dropw...
Embodiment 7
[0074] Preparation of N-[2-(7-methoxy-1-naphthyl)ethyl]acetamide (agomelatine)
[0075] Dissolve 40g of 2-(7-methoxy-1-naphthyl)ethylamine in 250ml of pyridine and heat to 40°C to completely dissolve. Under cooling and stirring in an ice bath, 21.9 g of acetyl chloride was slowly added dropwise. After dropping, remove the ice bath and stir at room temperature for 30 minutes. Then the reaction solution was poured into 300ml of ice water, while stirring vigorously, a large amount of white precipitate was separated out, and the stirring was continued for 1 hour, filtered, and the filter cake was washed with 200ml×2 water to obtain 48g of crude product (for the following preparation examples).
[0076] 1 H NMR (400MHZ, CDCl 3 ): δ7.77-7.15(m 6H,); δ5.61(s, 1H,); δ3.99(s, 3H,); δ3.62(m, 2H,); δ3.25(t, 2H ,); δ1.95(s, 3H,) is consistent with the literature report (J.Med.Chem, 1994, 37(20), 3231-3239.
[0077] Preparation Example
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com