Crystal structure for medicinal compound adefovir divoxil, and its preparing method and medicine
A technology of adefovir dipivoxil and crystal structure, applied in the field of nucleoside medicinal compounds, can solve the problems of high solvent toxicity, high cost of preparation of adefovir dipivoxil crystal form, unfavorable environmental protection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation method one of embodiment 1 adefovir dipivoxil crystal
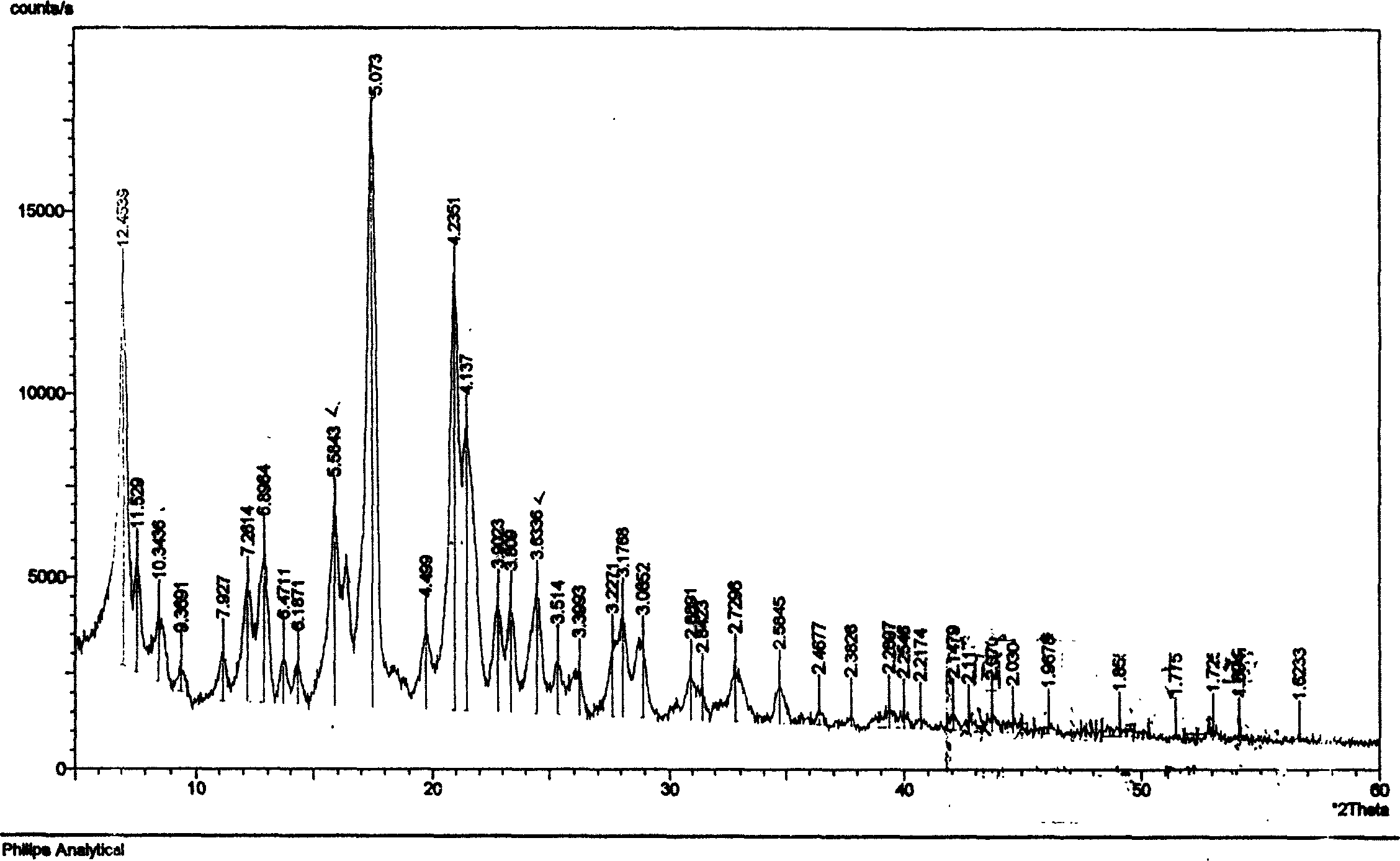
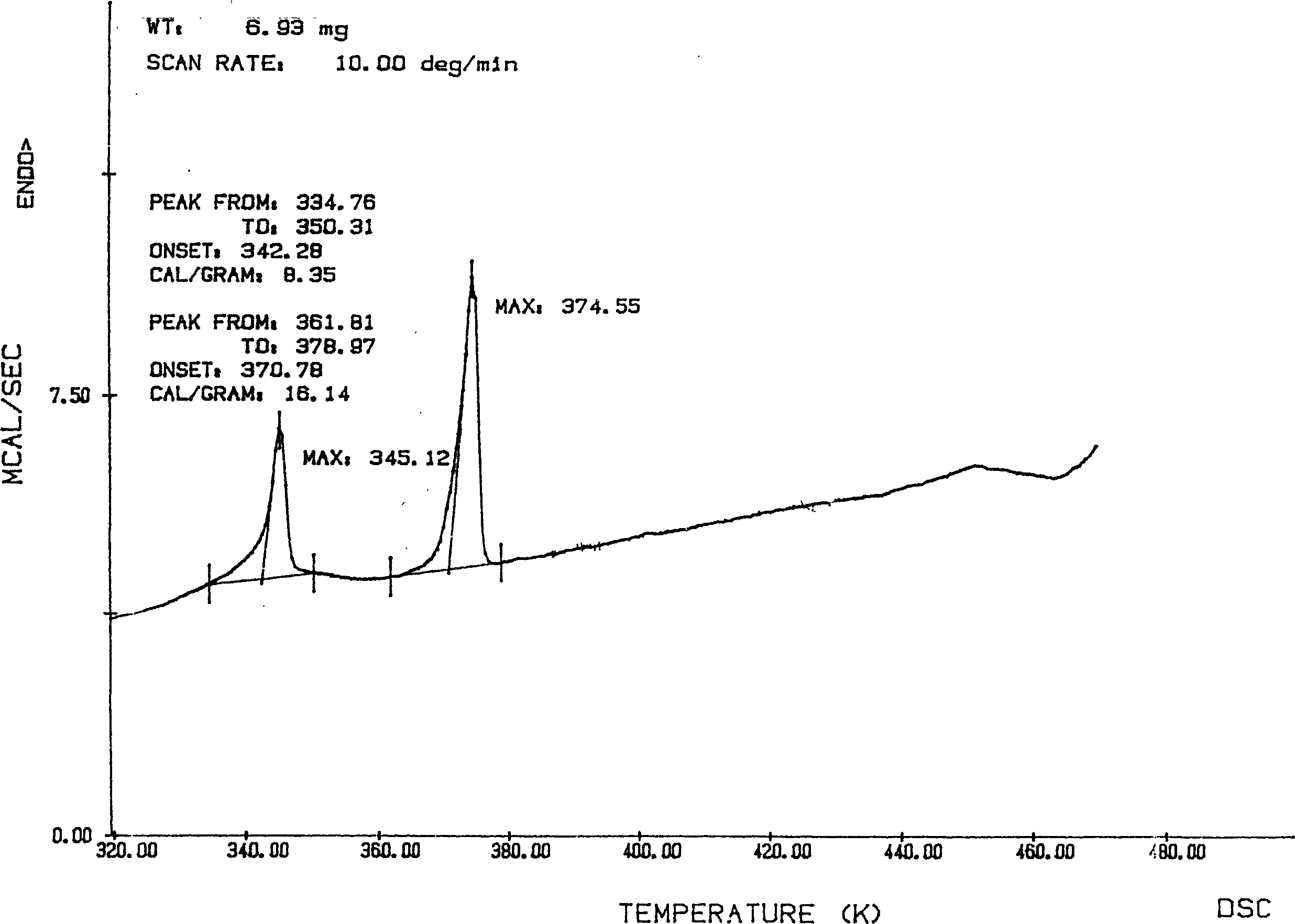
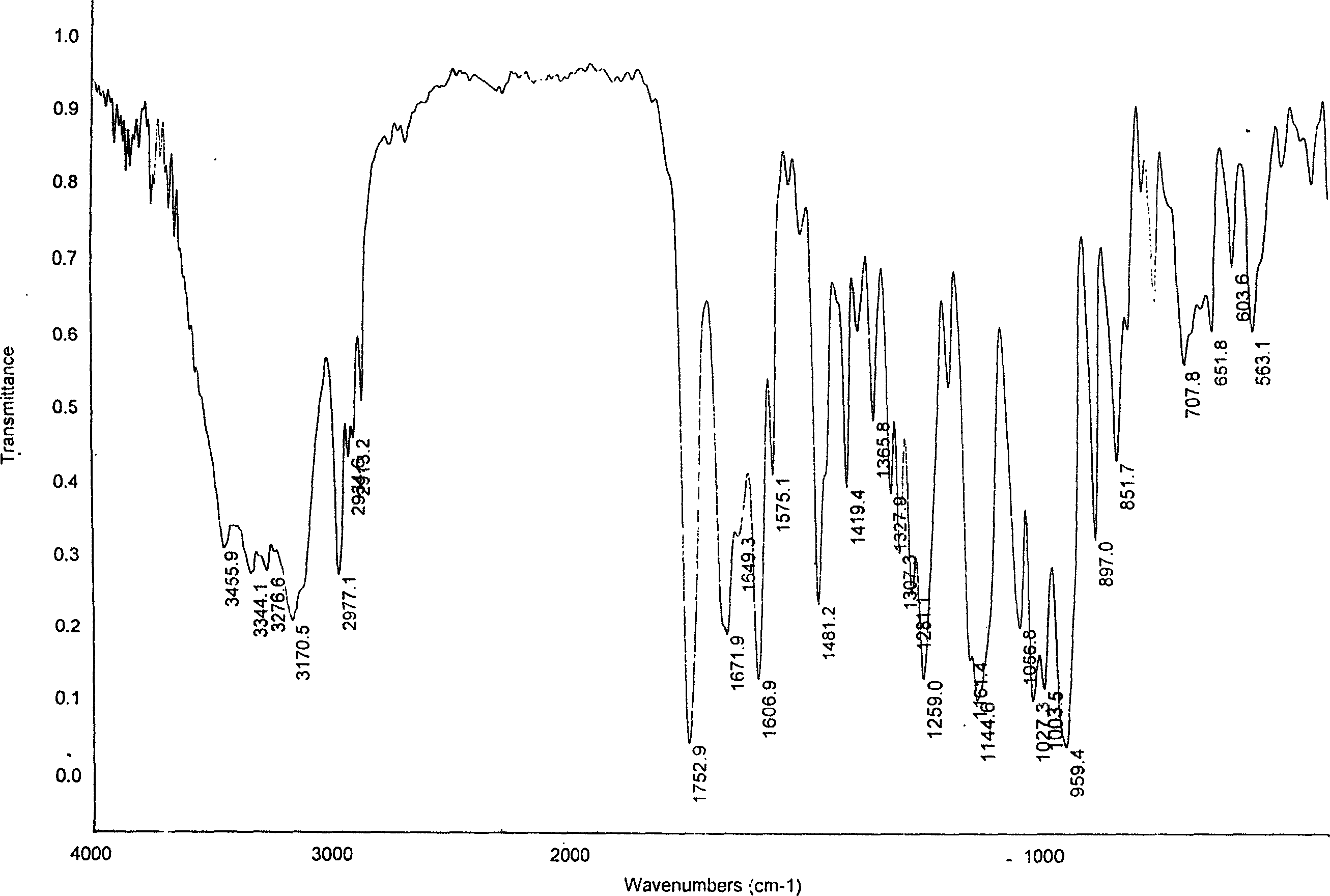
[0034] Take raw material adefovir dipivoxil crude product 31.0g, add 650ml of isopropyl acetate to dissolve, transfer the solution to a 2L separatory funnel, wash twice with 100ml×2 saturated saline, separate the liquids, add anhydrous sulfuric acid to the organic layer Dry magnesium, filter, concentrate to dryness under reduced pressure at 50°C, add 100ml of acetone to dissolve the solid, filter to remove insoluble matter, transfer the filtrate to a 250ml round bottom flask, heat at 60°C to reflux for 1h, and pour into 1000ml of ice-cold iso In propyl ether, stirring vigorously at the same time, a solid was precipitated, washed with cold isopropyl ether, and the filter cake was vacuum-dried at room temperature to constant weight to obtain 25.5 g of adefovir dipivoxil new crystal form, yield: 82%, content ≥ 99 % (HPLC). The X-ray powder diffraction spectrum, DSC collection of illustrative plates an...
Embodiment 2
[0035] Example 2 Preparation method two of the new crystal form of adefovir dipivoxil
[0036]Take 50.0 g of the raw material adefovir dipivoxil crude product, add 1000 ml of ethyl acetate to dissolve, transfer the solution to a 5 L separatory funnel, wash with 300 ml × 3 saturated saline three times, separate the layers, and dry the organic layer with anhydrous magnesium sulfate , filtered, concentrated to dryness under reduced pressure at 50°C, dissolved the solid with 300ml acetone, filtered to remove insoluble matter, transferred the filtrate to a 500ml round bottom flask, heated to reflux at 60°C for 1h, and poured into 1000ml ice-cold butyl ether while heating , while stirring vigorously, a solid was precipitated, washed with cold butyl ether, and the filter cake was vacuum-dried to constant weight at room temperature to obtain 42.5 g of adefovir dipivoxil new crystal form, yield: 85%, content ≥ 99% (HPLC) . The X-ray powder diffraction spectrum, DSC collection of illus...
Embodiment 3
[0037] Embodiment 3 Preparation method three of adefovir dipivoxil crystals
[0038] Take 62.0g of adefovir dipivoxil crude product, add 1000ml of ethyl acetate to dissolve, transfer the solution to a 5L separatory funnel, wash twice with 300ml×2 saturated saline, separate the layers, and dry the organic layer with anhydrous magnesium sulfate , filtered, concentrated to dryness under reduced pressure at 50°C, dissolved in 300ml of cyclohexanone for the solid, filtered to remove insoluble matter, transferred the filtrate to a 500ml round bottom flask, heated to reflux at 60°C for 1h, poured into 1000ml of ice-cold iso In propyl ether, stirring vigorously at the same time, a solid was precipitated, washed with cold isopropyl ether, and the filter cake was vacuum-dried at room temperature to constant weight to obtain 51.5 g of adefovir dipivoxil new crystal form, yield: 83%, content ≥ 99 % (HPLC). The X-ray powder diffraction spectrum, DSC collection of illustrative plates and I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com