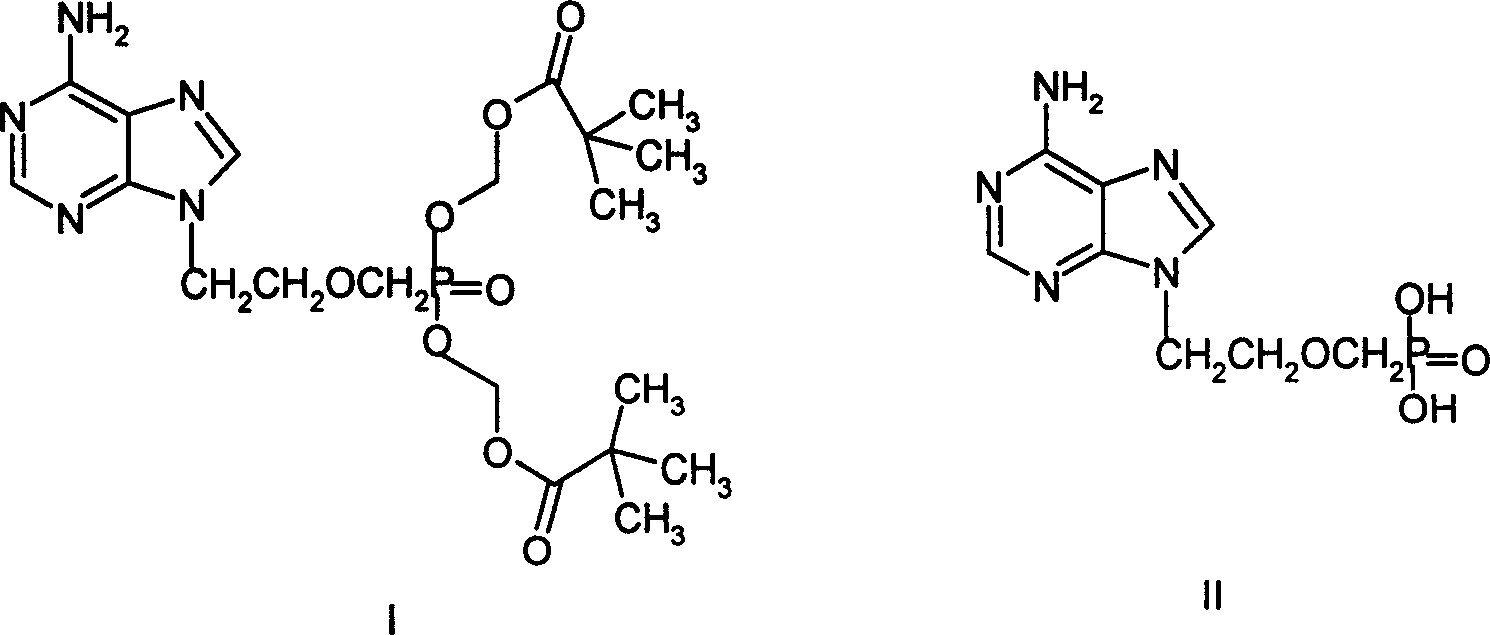
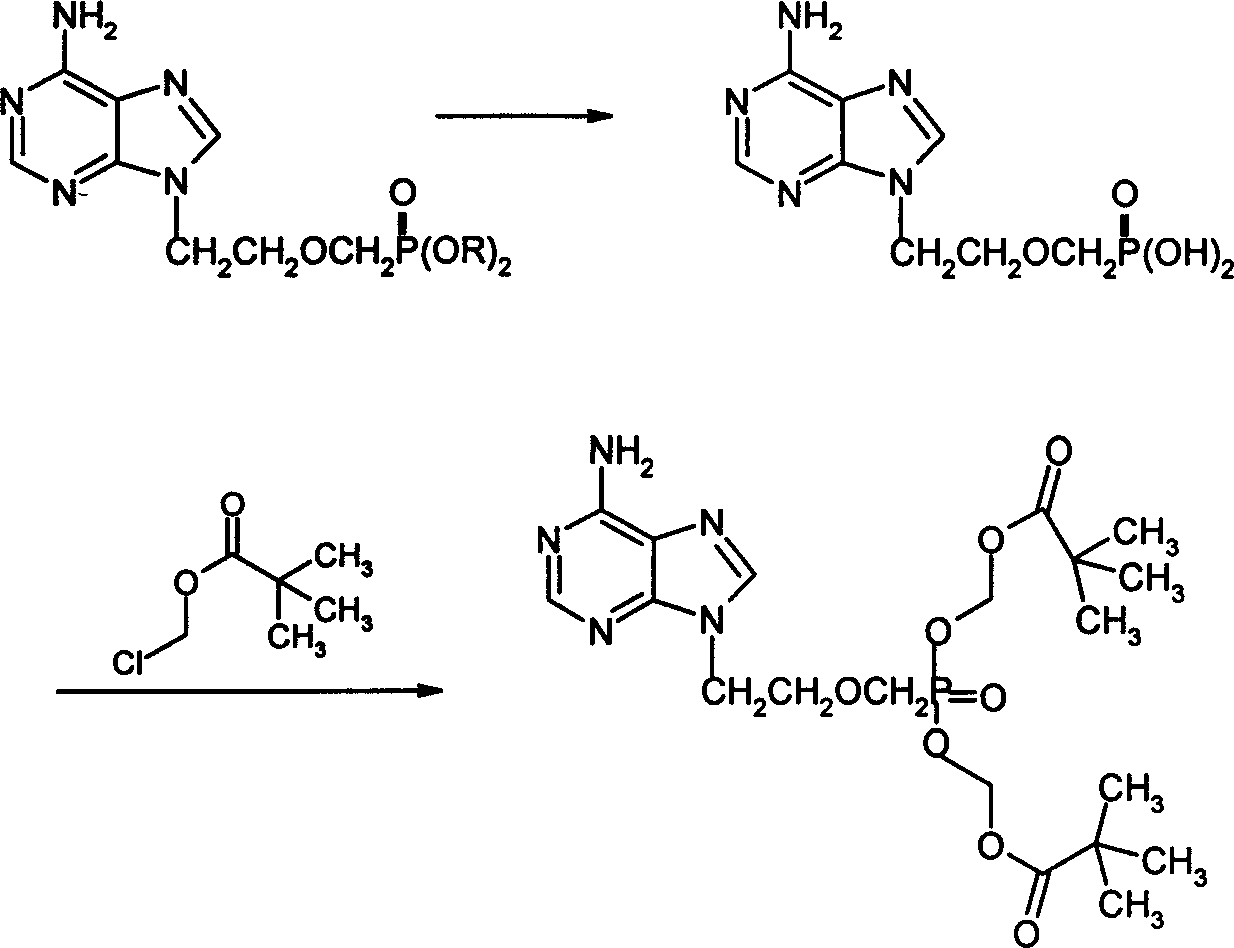
Synthesis process for Adefovir ester of anti hepatitis type B virus medicine
A technology of adefovir dipivoxil and a synthesis method, which is applied in the field of synthesis technology for preparing anti-hepatitis B virus drug adefovir dipivoxil, can solve problems such as being unsuitable for industrialized production, and achieve the advantages of improving yield, reducing cost and reducing pollution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
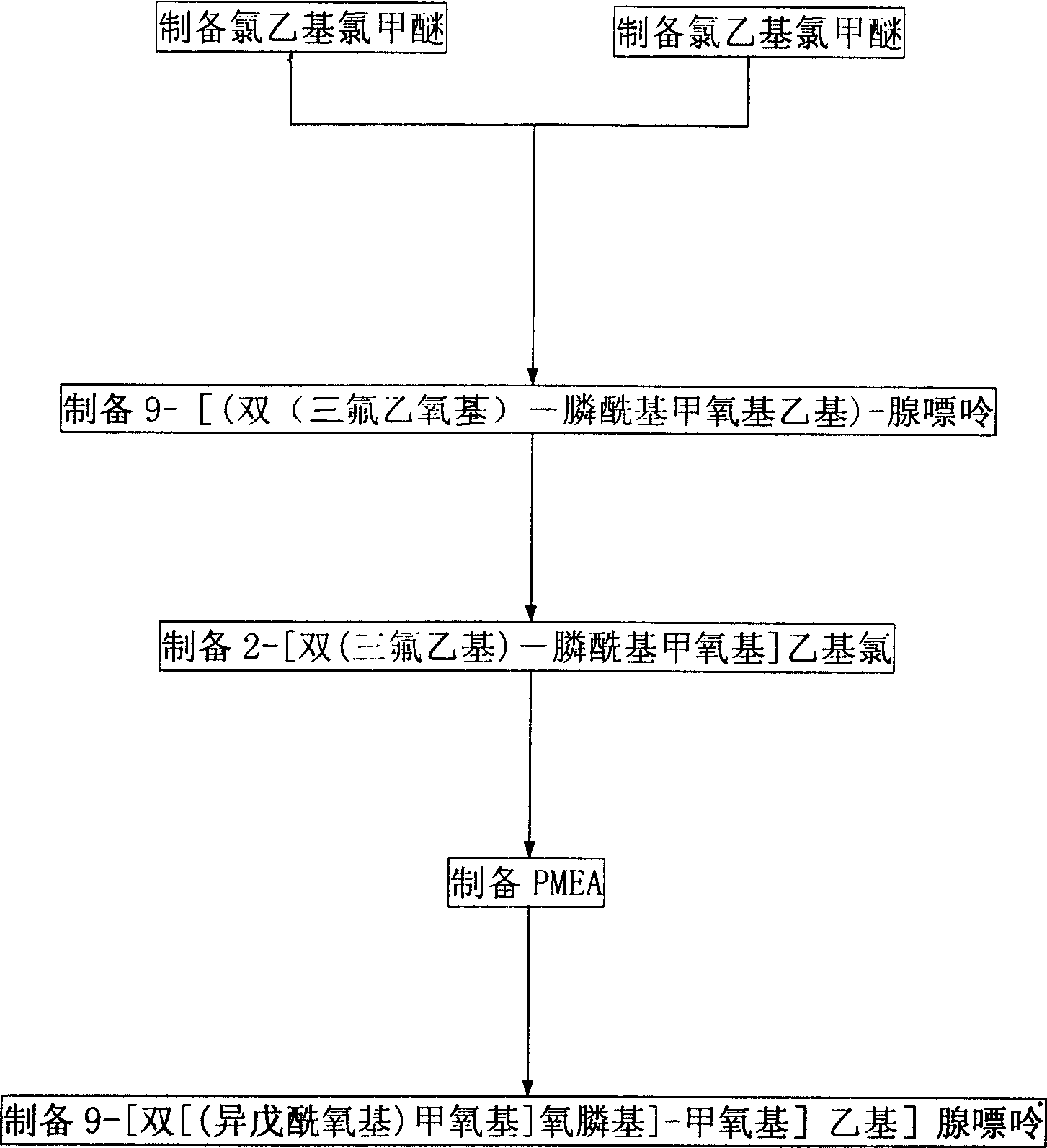
[0037] (1) Preparation of Chloroethyl Chloromethyl Ether (III)
[0038] In a 1500ml three-necked flask, add 846g (705ml, 10.5mol) of 2-chloroethanol and 316g (10.9mol) of pulverized paraformaldehyde, and pass in dry hydrogen chloride gas under stirring for 24 hours. After the stirring was stopped, the reaction solution was divided into two layers; the lower layer was separated and dried with calcium chloride. After filtration, the filtrate was fractionated under reduced pressure, and the fraction with a boiling range of 80-84° C. / 28-30 mmHg was collected to obtain 743 g of chloroethyl chloromethyl ether (III), with a yield of 54.9%.
[0039] (2) Preparation of three-(2,2,2-trifluoroethyl) phosphite (IV)
[0040] In 46 grams of trifluoroethanol, 21 grams of phosphorus trichloride was added, and the mixture was stirred and reacted at 80-90° C. for 4 hours. Fractional distillation yielded 41 grams of tris-(2,2,2-trifluoroethyl)phosphite (IV), 130-131°C / 74-78mmHg.
[0041] (3) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com