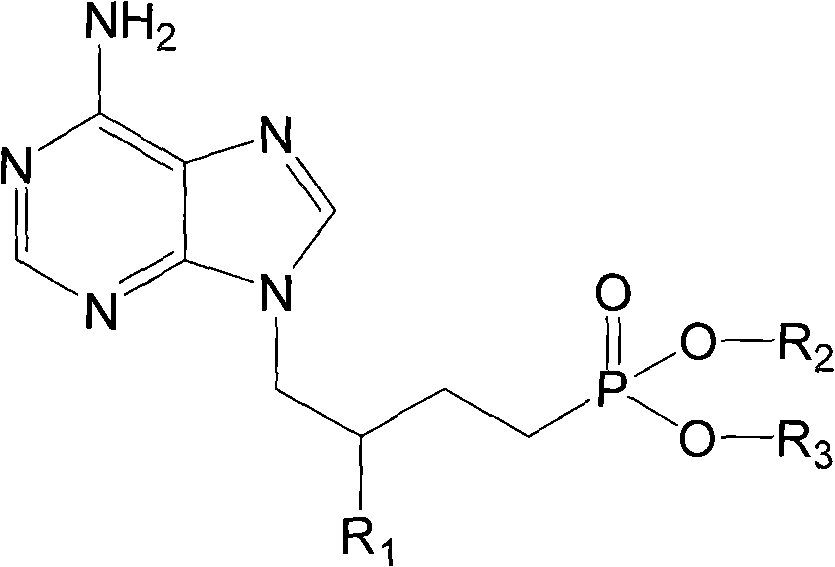
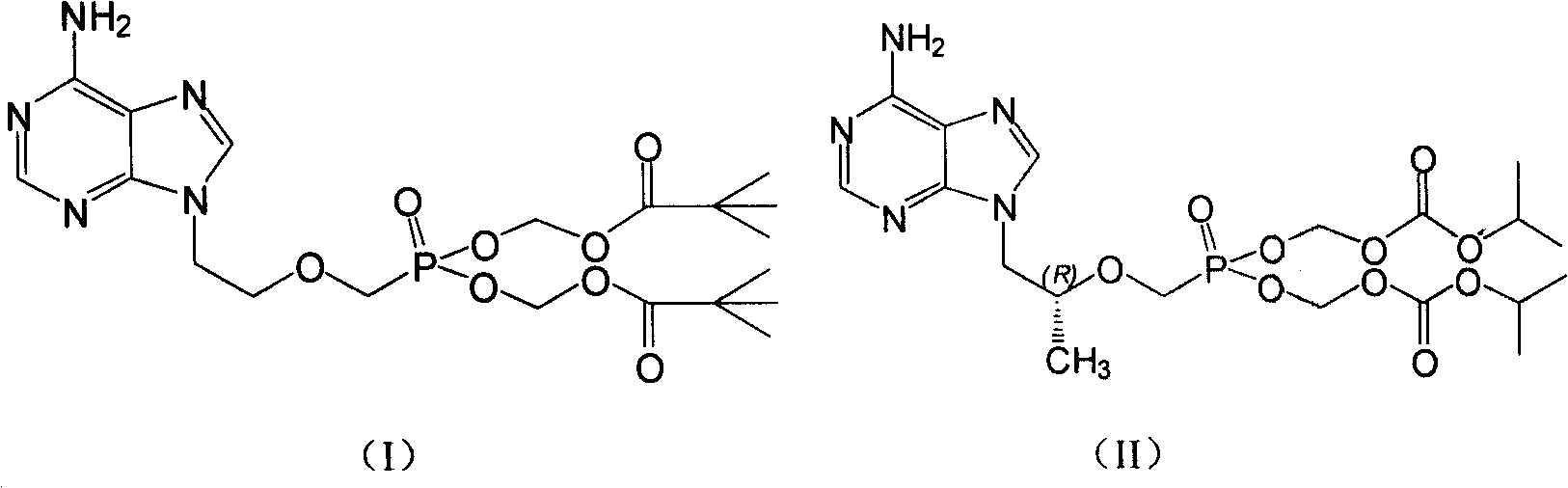
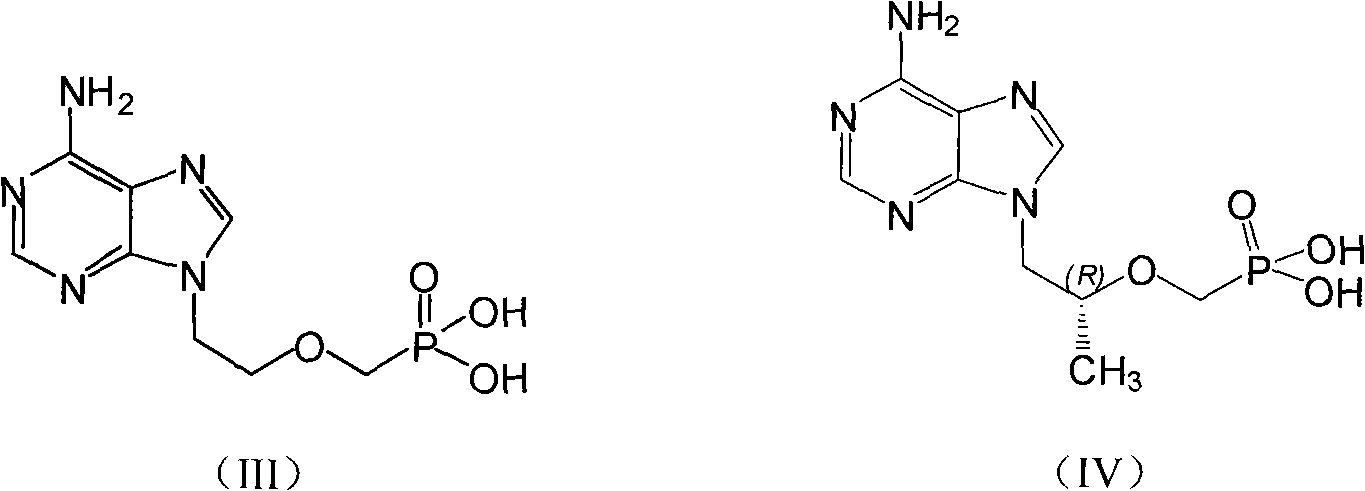
Prodrug with liver-targeted anti-HBV effect
An anti-hepatitis B, liver-targeting technology, applied in antiviral agents, medical preparations containing active ingredients, organic chemistry, etc. The effect of reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Example 1: Preparation and identification of adefovir dipalmitoyloxymethyl ester
[0102]
[0103] A 250mL three-neck flask was equipped with a stirrer, a condenser, and a thermometer, and 52g of palmitic acid was added, and the temperature was raised to 80°C to dissolve the solid, then a few drops of DMF was added, and 50mL of thionyl chloride was slowly added dropwise under stirring, and hydrogen chloride gas was released. After the addition, stir for another 2 hours to obtain a yellow clear liquid. Add 6 g of paraformaldehyde and 0.6 g of anhydrous zinc chloride, stir for 3 hours, and the paraformaldehyde gradually dissolves. The reaction solution was poured into ice water, and sodium hydroxide solution was added under stirring to make it neutral. The oil was extracted with ethyl acetate, the ester layer was washed with water, and dried over anhydrous sodium sulfate. Evaporate to dryness under reduced pressure and solidify to obtain 58 g of chloromethyl palmitate...
Embodiment 2
[0105] Example 2: Preparation and Identification of Tenofovir Dipalmitoyloxymethyl Ester
[0106]
[0107] In the same manner as in Example 1 above, tenofovir was used instead of adefovir to obtain an off-white solid.
[0108] MS-ESI(+): 824[M+H] + .
Embodiment 3
[0109] Example 3: Preparation and identification of adefovir monopalmitoyloxymethyl ester
[0110]
[0111] Add 1.0 g of adefovir, 1.1 g of chloromethyl palmitate, 20 mL of N-methylpyrrolidone and 1 g of DCC into a 100 mL round bottom flask, and stir and react at 50° C. for 3 hours. Add 50mL of ethyl acetate, extract with 1M sodium hydroxide solution, neutralize the extract with hydrochloric acid and then extract with ethyl acetate, dry the ester phase with anhydrous sodium sulfate, make sand, and elute with ethyl acetate / absolute ethanol The reagent was separated by column chromatography to obtain 0.4 g of off-white solid. MS-ESI(-): 540[M-H]- .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com