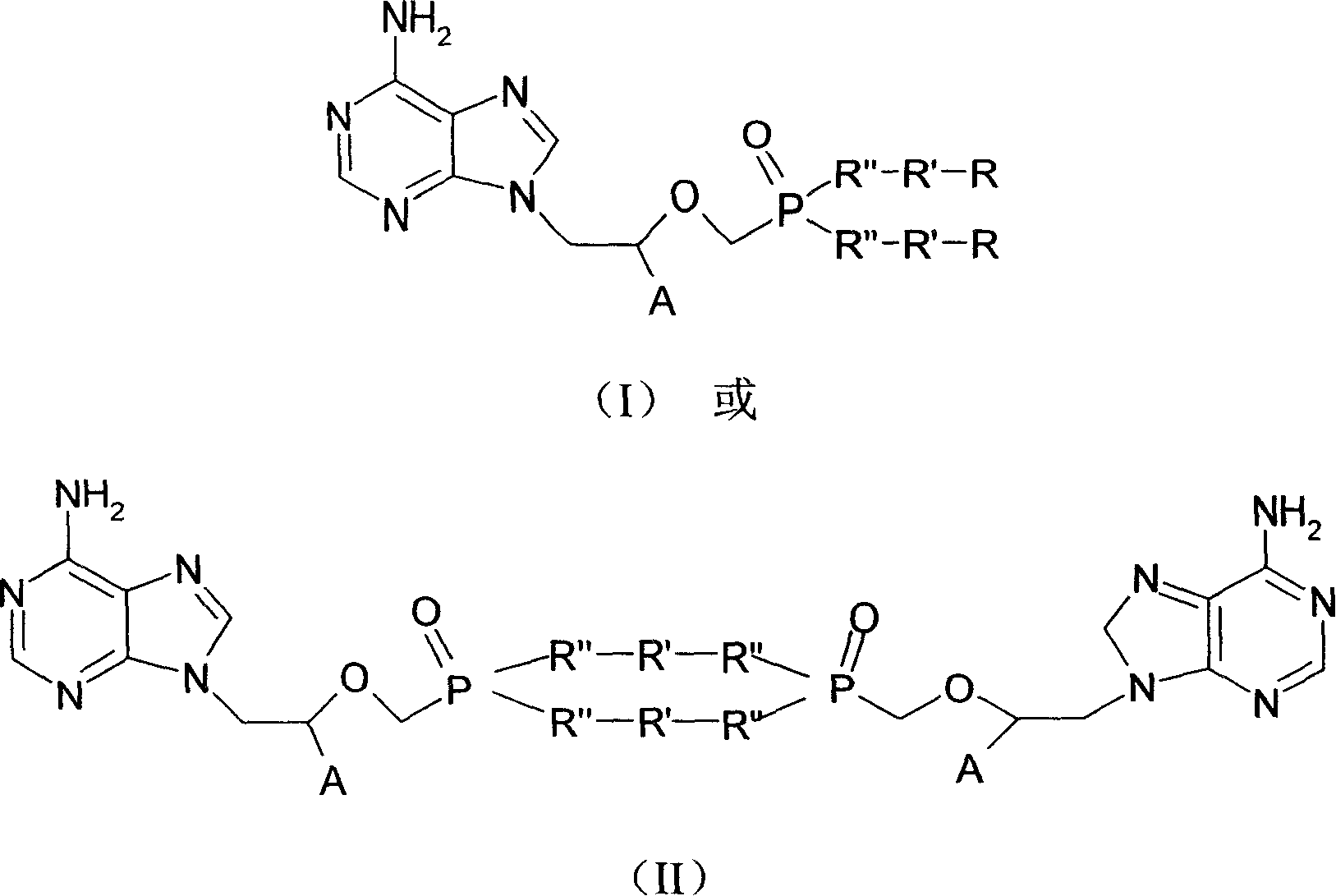
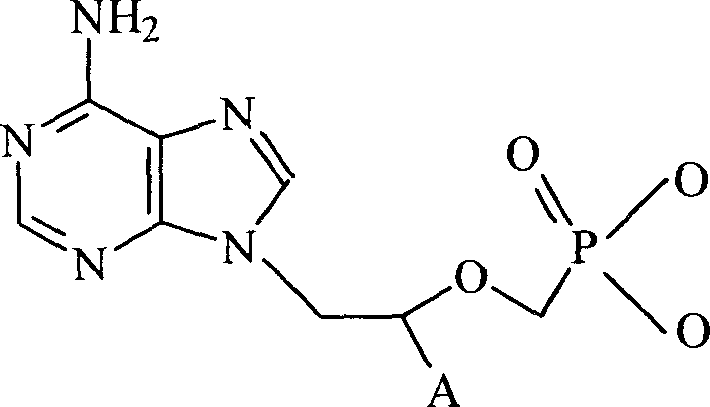
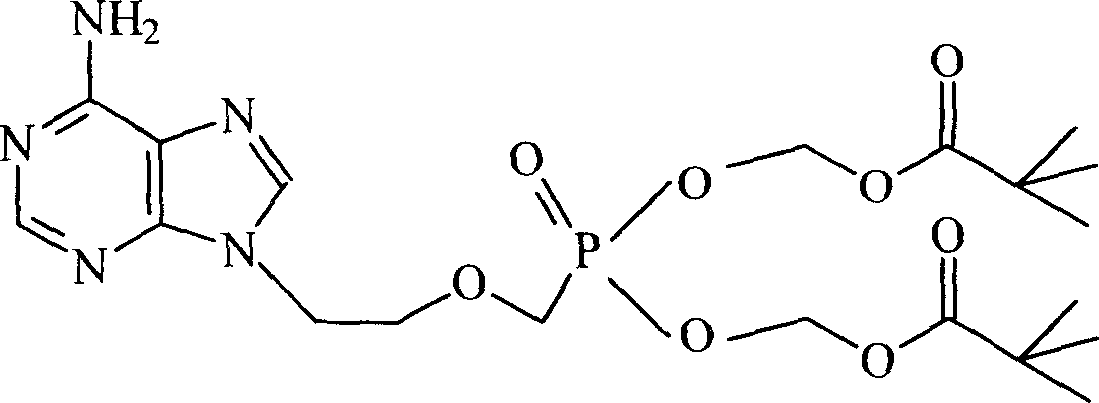
Chemical modified adefovir and tynofovir
A technology of compounds and derivatives, applied in the field of chemically modified adefovir and tenofovir, can solve the problems of slow phosphorylation of host cells and ineffective concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050]The preparation of embodiment 1 adefovir-methoxypolyethylene glycol 2000
[0051] Add 140g (0.07mol) of monomethoxypolyethylene glycol 2000 (mPEG2000) into 500ml of toluene, heat and melt, evaporate about 400ml of toluene, add 250ml of N,N-dimethylformamide, 70ml of pyridine, 9.6 g (0.035mol) adefovir and 27g dicyclohexylcarbodiimide, heat up to 100°C, and react at this temperature for 120 minutes, cool to room temperature, add 1000ml ethyl acetate, stir for 2 hours, filter, The solvent was recovered from the mother liquor, 500ml of absolute ethanol was added to the residue to dissolve, and then slowly added into 2000ml of anhydrous diethyl ether with vigorous stirring, cooled, filtered, and the filter cake was dried under reduced pressure at room temperature for 24 hours to obtain 130g. Melting point 49~52℃, containing 6.8% adefovir.
Embodiment 2
[0052] Embodiment 2 Preparation of Adefovir-methoxypolyethylene glycol amine 2000
[0053] 140g (0.07mol) polyethylene glycol amine 2000 (mPEG-NH 2 ) into 250ml of N,N-dimethylformamide, add 9.6g (0.035mol) of adefovir and 21g of DCM, stir at room temperature for 24 hours, add 1000ml of ethyl acetate, stir for 2 hours, filter, and recover the solvent from the mother liquor , 500ml of absolute ethanol was added to the residue to dissolve, then slowly added into 2000ml of anhydrous ether which was stirred vigorously, cooled, filtered, and the filter cake was dried under reduced pressure at room temperature for 24 hours to obtain 126g of product. Melting point 49~53℃, containing 6.8% adefovir.
Embodiment 3
[0054] Embodiment 3 Preparation of Adefovir-methoxypolyethylene glycol 1000
[0055] Prepared according to the method of Example 1, the molar ratio of monomethoxypolyethylene glycol 1000 and adefovir was 2:1, and adefovir polyethylene glycol 1000 was obtained, with a melting point of 34-38 ° C, containing adefovir Foway 13.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com