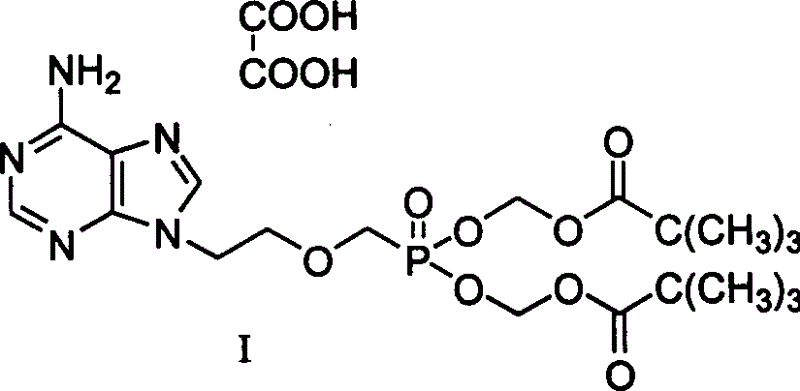
Oxalic adefovir dipivoxil, and crystalline form and preparing method and use thereof
A technology of adefovir dipivoxil and fovir dipivoxil, which is applied in the field of medicine, can solve problems not involving adefovir dipivoxil, etc., and achieve the effects of easy preparation, good fluidity, and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
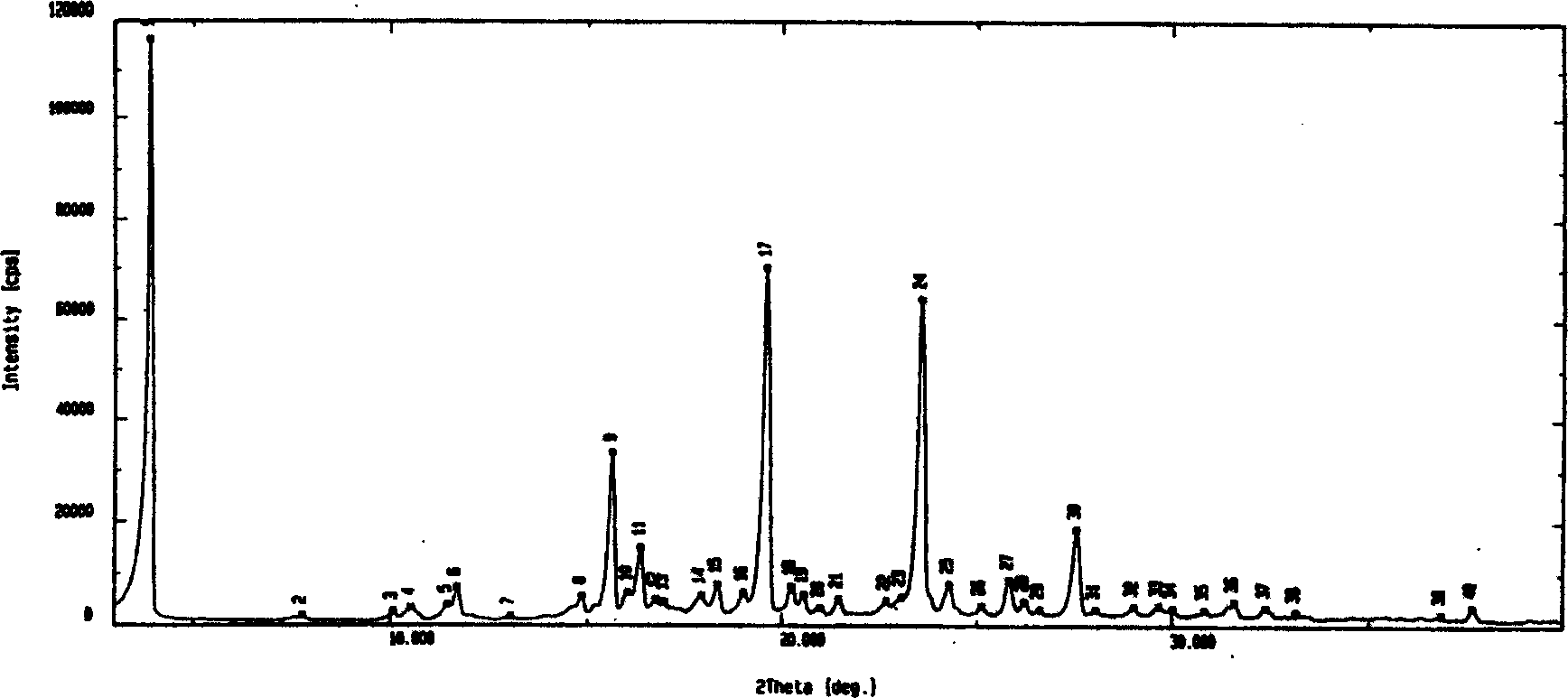
Image
Examples
Embodiment 1
[0022] The preparation of embodiment 1 adefovir dipivoxil oxalate crystal form
[0023] Add 10g of adefovir dipivoxil oil to 50mL of acetone, stir at room temperature to dissolve, add dropwise 1.8g of oxalic acid in 20mL of acetone solution, complete, stir at room temperature, and slowly precipitate a white crystalline solid, and place it overnight at 0-5°C. Suction filtration, wash with acetone until neutral, and dry to obtain 10.6 g of adefovir disoproxil oxalate as an off-white powder with a yield of 90%.
[0024] Melting point: 173~175℃
[0025] DSC: 175°C
[0026] Elemental analysis:
[0027] Found C 44.73%, H 5.79%, N 11.64
[0028] Theoretical value (C 20 h 32 N 5 o 8 P.C. 2 o 4 h 2 ) C 44.67%, H 5.75%, N 11.84
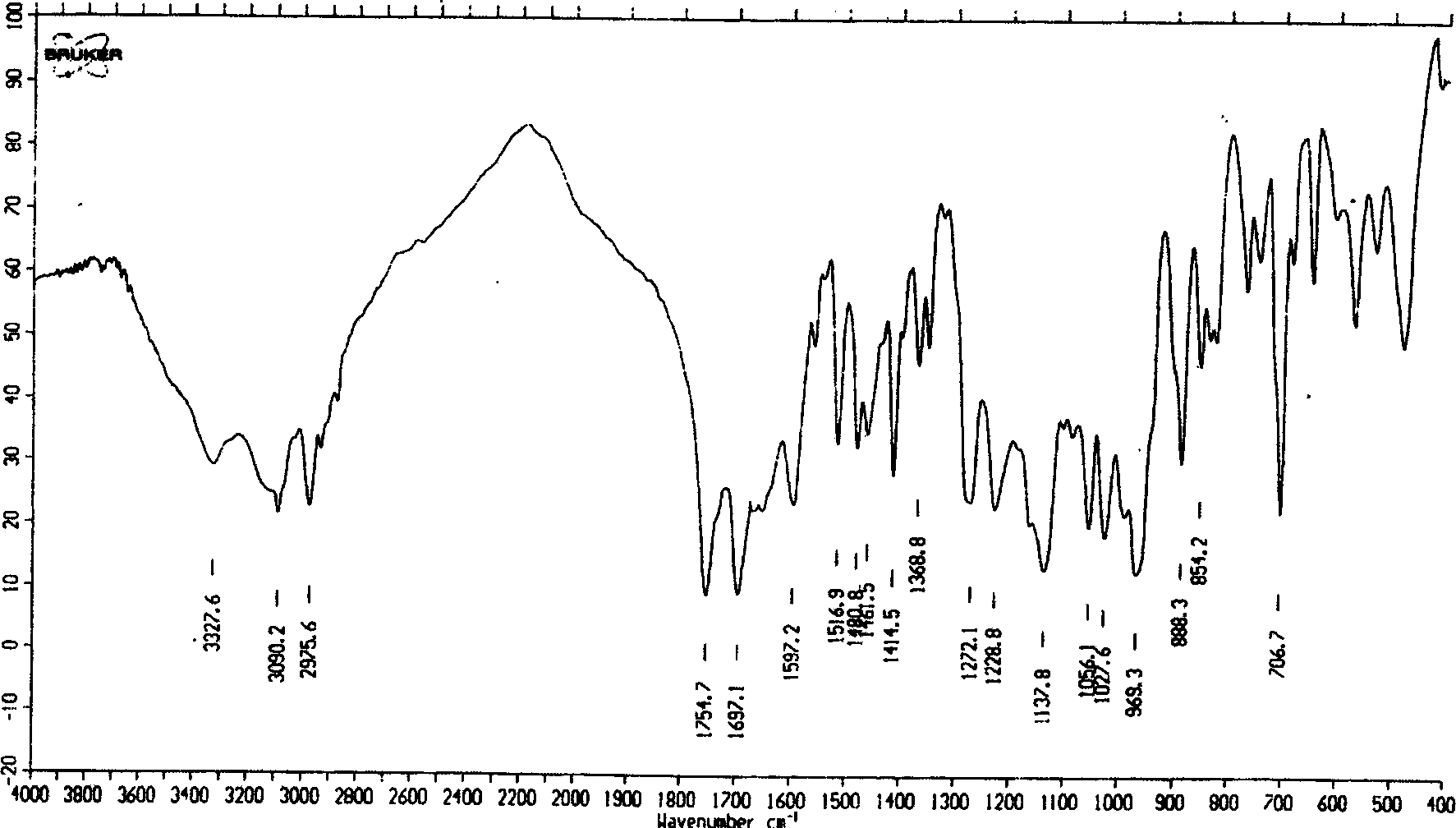
[0029] Properties of the crystal form of adefovir disoproxil oxalate
[0030] (1) Infrared absorption spectrum (attached figure 1 )
[0031] At 3090, 2975, 1754, 1697, 1516, 1480, 1461, 1414, 1271, 1228, 1137, 1056, 1027, 969, 888, 706cm -1
[0...
Embodiment 2
[0049] The preparation of embodiment 2 adefovir dipivoxil oxalate crystal form
[0050] Methanol was used to replace the acetone in Example 1, and the others were the same as in Example 1 to obtain adefovir dipivoxil with a yield of 80%.
Embodiment 3
[0051] The preparation of embodiment 3 adefovir dipivoxil oxalate crystal form
[0052] Acetonitrile was used instead of acetone in Experimental Example 1, and the others were the same as in Example 1 to obtain adefovir dipivoxil with a yield of 85%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com