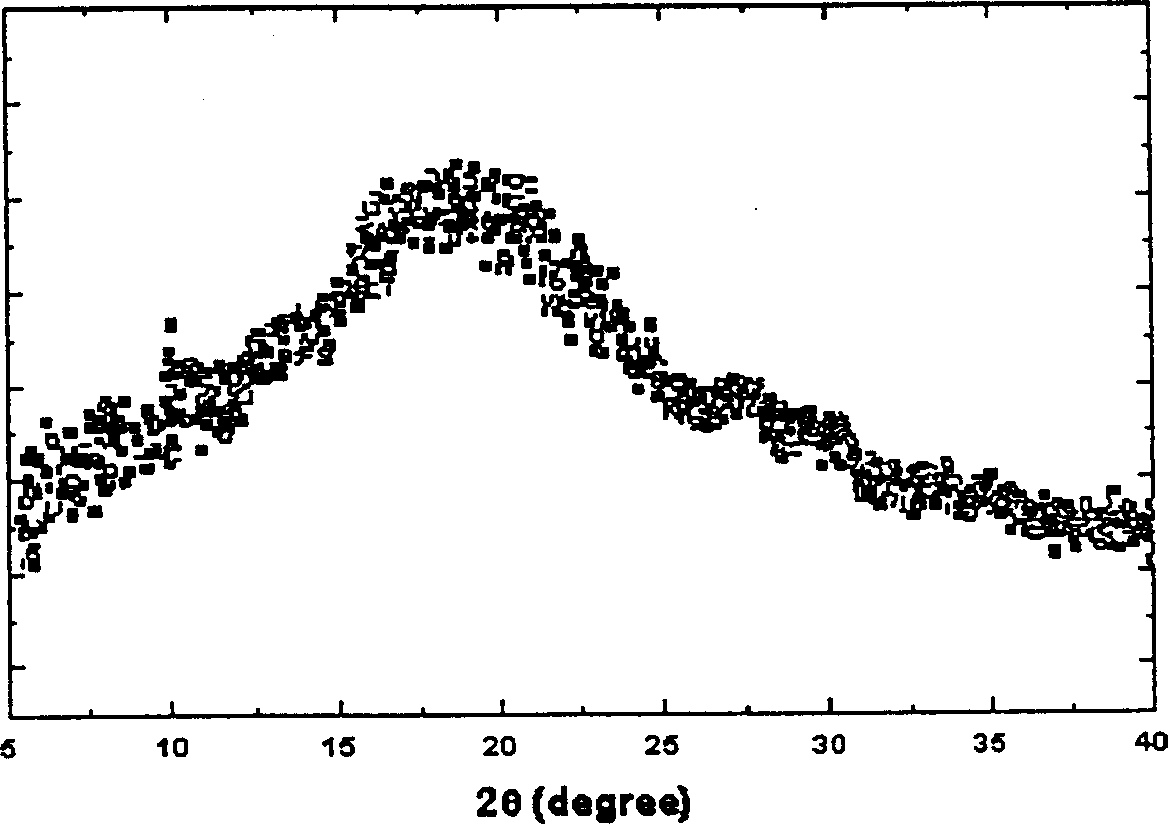
Amorphous Adefuweizhi ester amorphous solid matter and its prepn
Adefovir dipivoxil and amorphous technology, which is applied in the field of adefovir dipivoxil amorphous solidified products and its preparation, can solve the problems of high cost, cumbersome operation, difficult medicine, etc., and achieve low cost, convenient operation, manufacturing and Formulated for easy effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Adefovir dipivoxil oil (5g) was added to a lyophilized vial, and ethanol (10ml) was added to dissolve at 15-20°C, then cooled to -75°C and rotated to solidify. Maintain -60~-50℃ for vacuum freeze-drying, and in the final stage, the temperature can be raised to -10~0℃ for drying. About 93% of the obtained adefovir dipivoxil amorphous cured product exists in an amorphous form.
Embodiment 2
[0016] Using chloroform (5ml) instead of ethanol (10ml), the others were the same as in Example 1 to obtain an amorphous solidified product of adefovir dipivoxil, of which about 90% existed in an amorphous form.
Embodiment 3
[0018] Adefovir dipivoxil oil (5 g) was added to a lyophilized vial, and benzene (10 ml) was added to dissolve at 15-20° C., then cooled to -10° C. and rotated to solidify. Maintain vacuum freeze-drying at -10-0°C, and heat up to 5-10°C for drying in the final stage. About 95% of the obtained adefovir dipivoxil amorphous cured product exists in an amorphous form. Example 4
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com