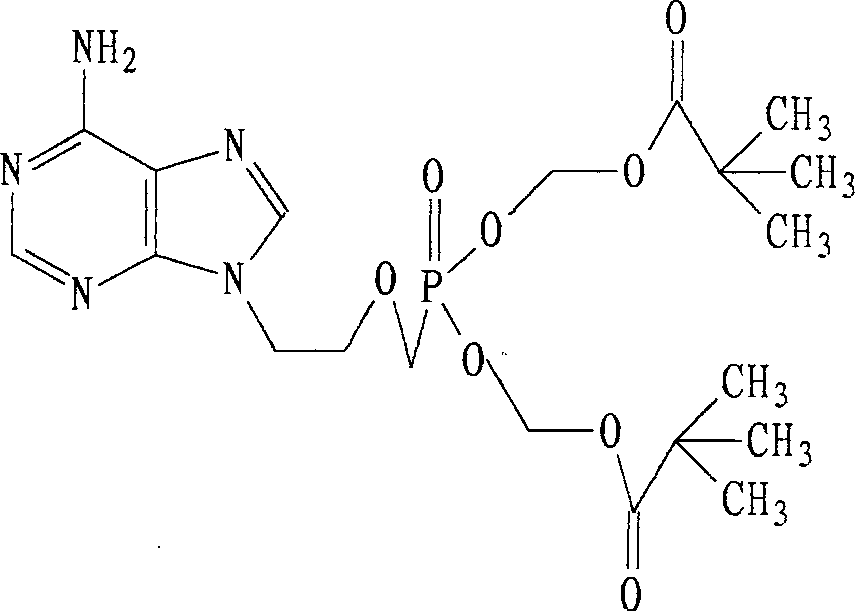
Crystalline form of Adefovir dipivoxil and preparing process thereof
A technology of adefovir dipivoxil and new crystallization, applied in the field of organic chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
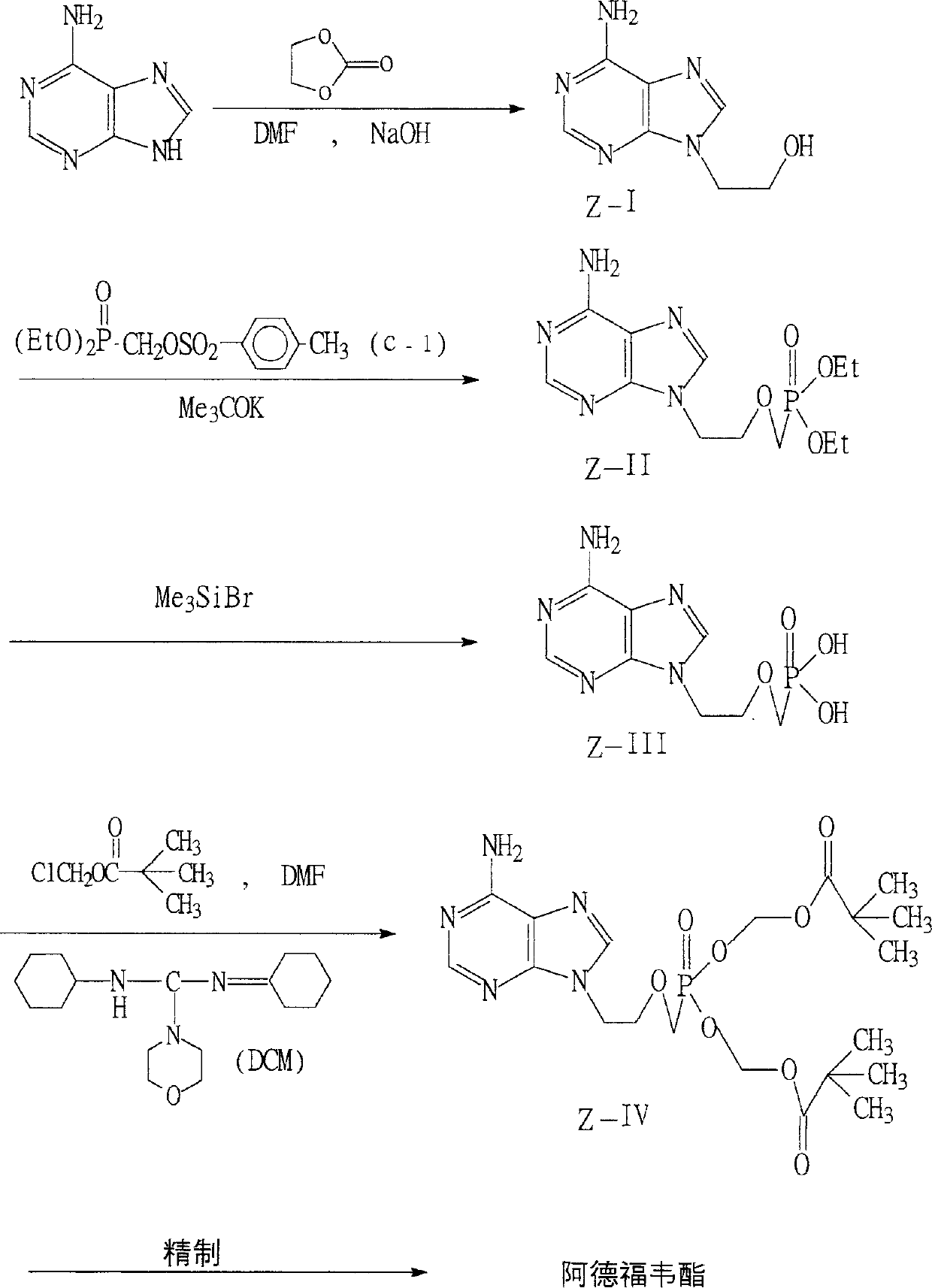
[0053] DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS The following examples are further illustrations of the present invention rather than limitations. Embodiment 1: Preparation of form 1 crystal
[0054] Referring to WO9904774 (CN1251592A) and EP481214 Example 8
[0055] (1) Diethyl p-toluenesulfonyloxymethylphosphonate (C-1)
[0056] Add 500g (3.6mol) diethyl phosphite, 137.5g (4.5mol) paraformaldehyde, 76g triethylamine and 2100ml toluene into a 5000ml three-necked flask, heat to 85°C (85-105°C), stir for 2h, then Reflux for 2h. The temperature was lowered to 0°C and 625g of p-toluenesulfonyl chloride was added, and 474g of triethylamine was added dropwise at 0°C. After addition, stir at room temperature for 10h. Filter and wash the filter cake with 100ml×3 toluene. Combine the organic phases, successively use 700ml water, 2000ml 5% Na 2 CO 3 Aqueous solution, washed with 2000ml water. The organic layer was separated, and the solvent wa...
Embodiment 2
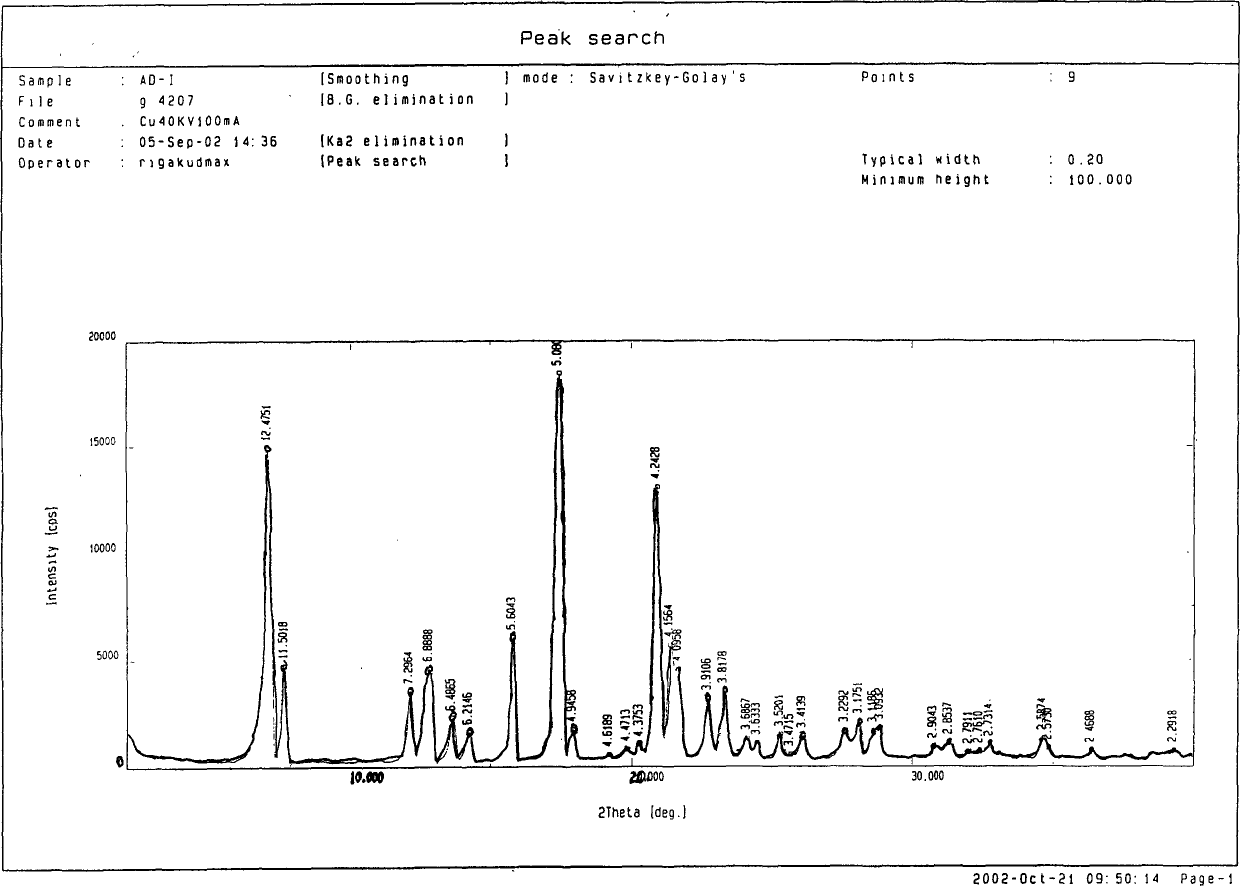
[0068] Form 1 Dissolve 50g of adefovir dipivoxil in 300mL of acetone to obtain a colorless and transparent solution. Evaporate the solvent to dryness in vacuo at 30-40°C to obtain an oil, gradually cool to room temperature, solidify into a white solid, grind, and dry in vacuo at 60°C After 8 hours, 49 g of a white powdery solid was obtained, which was adefovir dipivoxil in a new crystal form. Embodiment 3: the preparation of the adefovir dipivoxil of new crystal form
Embodiment 3
[0069] Form 1 Adefovir dipivoxil 50g was dissolved in 500mL isopropanol to obtain a colorless and transparent solution. The solvent was evaporated to dryness in vacuo at 30-60°C to obtain an oil, which was gradually cooled to 30°C and solidified into a white solid. Grinding, 60 ℃ vacuum drying for 8 hours to obtain 50 g of white powdery solid, which is adefovir dipivoxil in a new crystal form. Embodiment 4: the preparation of the adefovir dipivoxil of new crystal form
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com