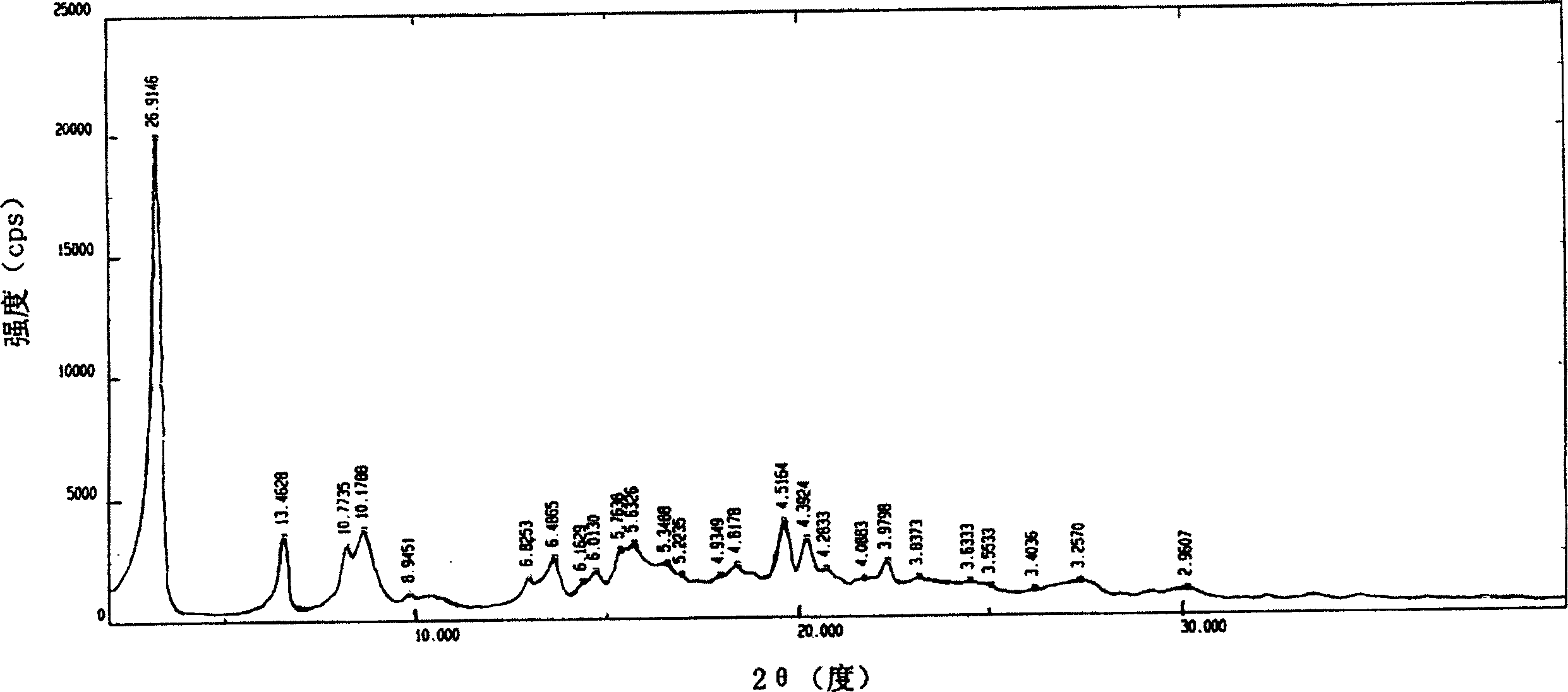
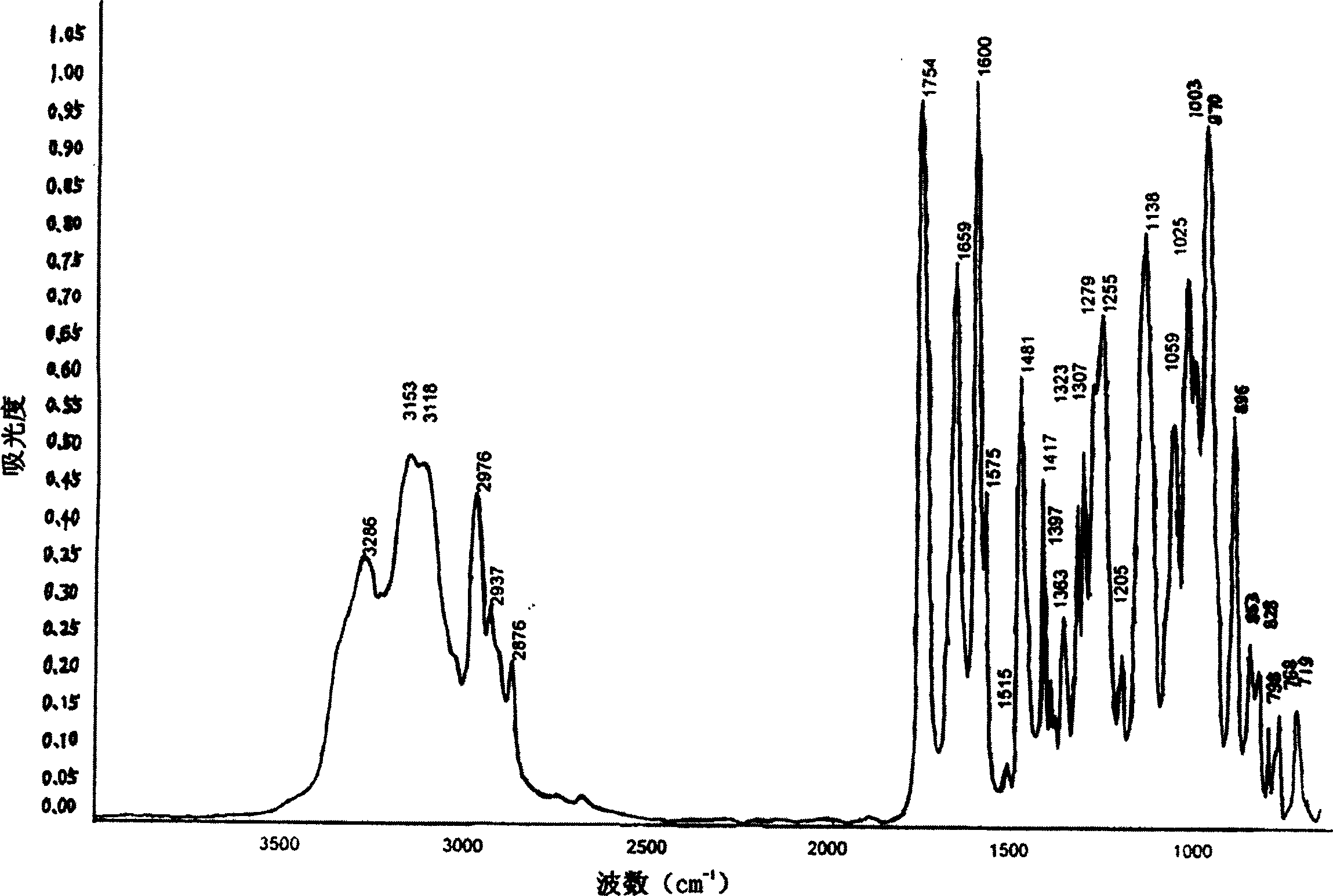
Adefovir dipivoxil new crystal state, new crystal state composition and its preparing method
A technology of adefovir dipivoxil and composition, applied in the field of new crystal state of nucleoside drugs, new crystal state composition and preparation thereof, can solve the problems of high cost, harsh conditions, unfavorable large-scale industrial manufacturing and the like, To achieve the effect of no reduction in drug content, easy production and good crystal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 Alcohol method prepares adefovir dipivoxil new crystal state
[0021] Add 50g of adefovir dipivoxil into a 500ml flask, add 200ml of absolute ethanol at 20-30°C to dissolve it, and spin dry the solvent under reduced pressure at 50°C until it becomes a colorless oil, then rapidly heat up to 80 ℃, the oil can quickly expand into a honeycomb. After all the remaining solvents were volatilized, it was then vacuum-dried at 50° C. for 5 hours to obtain a new crystalline form of adefovir dipivoxil with a purity of 90%.
Embodiment 2
[0022] Embodiment 2 ketone method prepares adefovir dipivoxil new crystal state
[0023] Add 50g of adefovir dipivoxil into a 500ml flask, add 300ml of acetone at 20-30°C to dissolve it, spin-dry the solvent under reduced pressure at 30°C until it becomes a colorless oily substance, then rapidly heat up to 60°C, The oil can quickly expand into a honeycomb. After all the residual solvents were volatilized, it was then vacuum-dried at 50° C. for 5 hours to obtain a new crystalline form of adefovir dipivoxil with a purity of 85%.
Embodiment 3
[0024] Embodiment 3 ester method prepares adefovir dipivoxil new crystal state
[0025] Add 50g of adefovir dipivoxil into a 500ml flask, add 400ml of ethyl acetate at 20-30°C to dissolve it, and other operations are the same as in Example 2 to obtain a new crystalline form of adefovir dipivoxil with a purity of 88%. .
[0026] Embodiment 4 halogenated hydrocarbon method prepares adefovir dipivoxil new crystal state
[0027] Add 50g of adefovir dipivoxil into a 500ml flask, add 200ml of chloroform at 20-30°C to dissolve it, and other operations are the same as in Example 2 to obtain a new crystalline form of adefovir dipivoxil with a purity of 88%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com