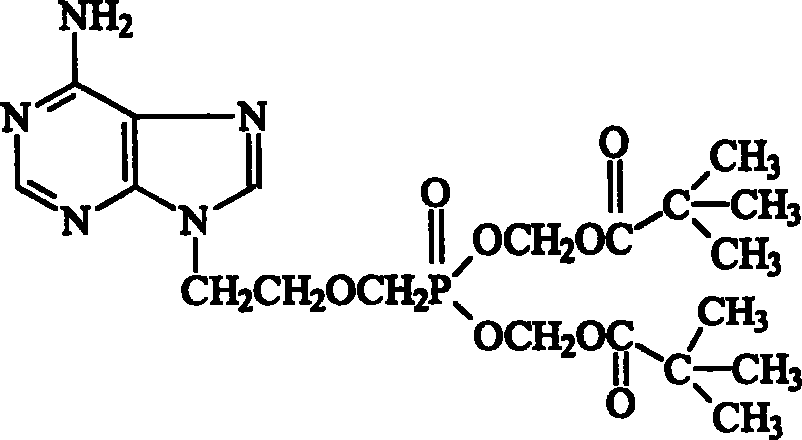
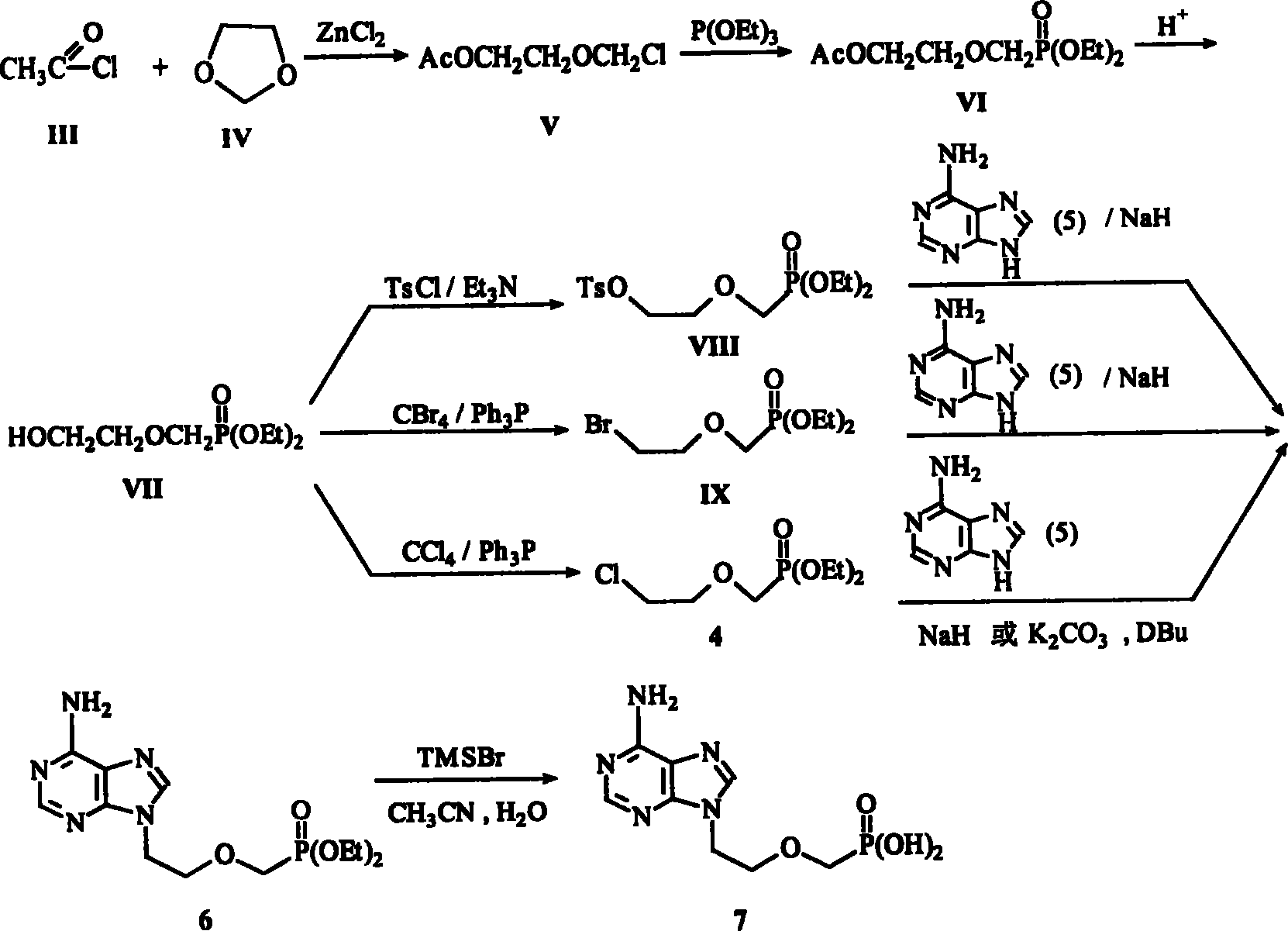
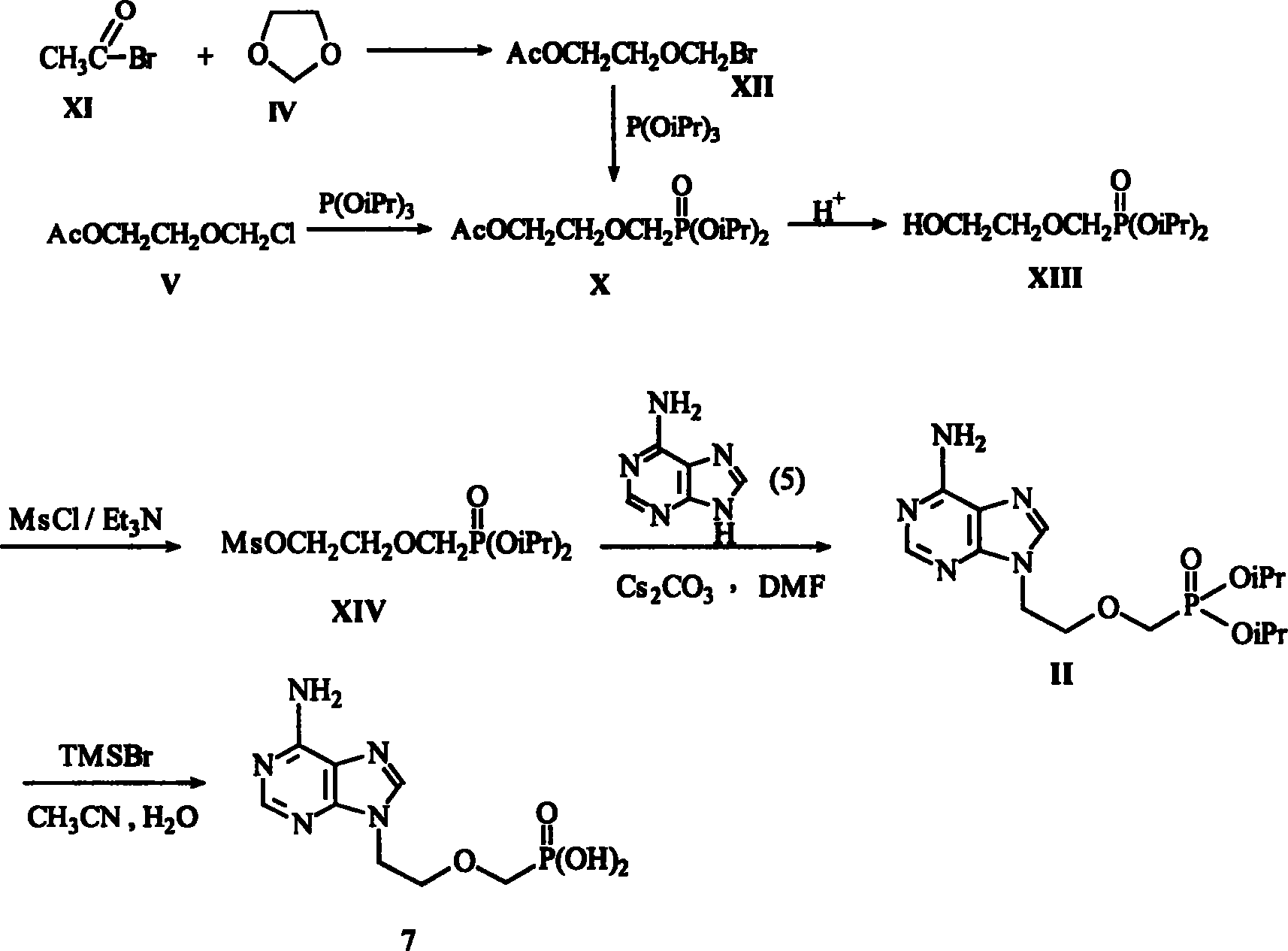
Method for synthesizing adefovir dipivoxil ester
A technology of adefovir dipivoxil and synthetic method, which is applied in the field of drug synthesis, can solve the problems of low yield, complex process, and long reaction steps, and achieve high total yield, high yield, and short synthetic route steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 1. Synthesis of 1-chloro-2-chloromethoxyethane (3)
[0057] Feeding amount:
[0058]
[0059] Steps:
[0060] Suspend 30.0 g of paraformaldehyde (2) in 80.5 g of 2-chloroethanol (1), stir at room temperature, pass through anhydrous hydrogen chloride gas for 22 hours, the solid is dissolved, and the water layer is separated. The organic layer was dried by adding 20.0 g of anhydrous calcium chloride, passed through nitrogen for 4 h, filtered and distilled under reduced pressure, and 90.6 g of bp62-66°C / 29Mg light yellow transparent liquid (3) was collected with a yield of 70.2%. Quality control method: bp62-66℃ / 29mmHg.
[0061] Attachment: Preparation of anhydrous hydrogen chloride gas: Mix concentrated hydrochloric acid and sodium chloride evenly, slowly add concentrated sulfuric acid dropwise, and the generated hydrogen chloride gas is dried by concentrated sulfuric acid.
[0062] 2. Diethyl 2-chloroethoxymethylate (4)
[0063] Feeding ratio
[0064]
[0065]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com