Kit for genotyping VZV, production method of kit and application of kit
A varicella zoster and kit technology, which is applied in the field of kits for typing and detecting varicella zoster virus, which can solve the problems of difficult differential diagnosis, incapable of high-throughput detection, incapable of batch detection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] The sources and records of the virus strains used for typing in Example 1 are shown in Table 2 below.
[0082] Table 2
[0083]
Embodiment 3
[0084] The sample herpes fluid to be tested used in the clinical verification of Example 3 was collected from patients with chickenpox and herpes zoster admitted to the General Hospital of the Chengdu Military Command of the Chinese People's Liberation Army.
[0085] Embodiment 1, the preparation of kit of the present invention
[0086] 1. Selection of varicella-zoster virus detection and typing sites
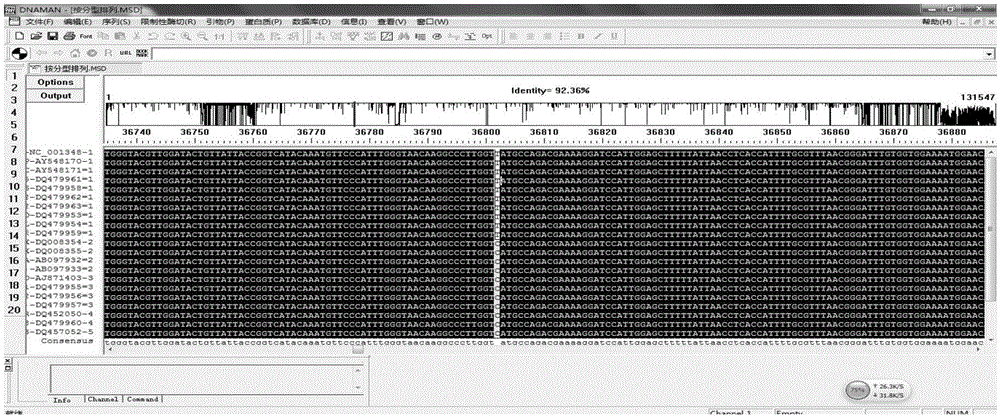
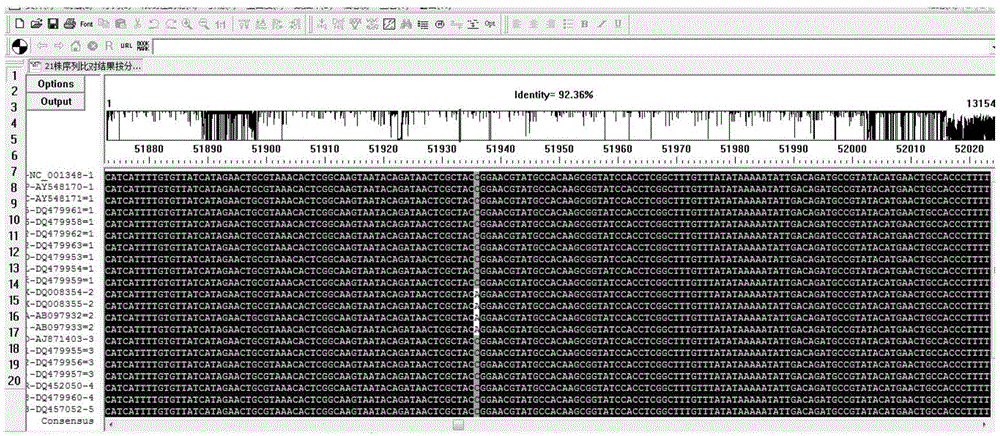
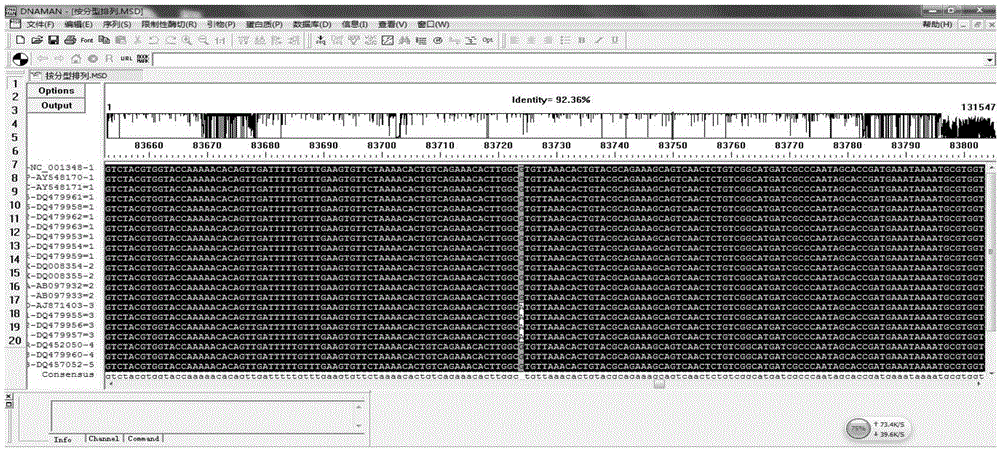
[0087] Using the NCBI nucleic acid database ( http: / / www.ncbi.nlm.nih.gov / nuccore / ) search and download the genome sequence of each virus strain of VZV, and type each virus strain according to the Clade typing method. Using DNAman software to conduct multiple sequence comparisons of VZV strains, herpes simplex virus type 1 (HSV1), herpes simplex virus type 2 (HSV2), cytomegalovirus (CMV), Epstein-Barr virus (EBV) strains, and human genome DNA sequences Yes, exclude homologous sequences and find out the specific SNP sites of various VZV strains.
[0088] A total of 21 strai...
Embodiment 2
[0104] Embodiment 2, the verification of kit sensitivity and specificity of the present invention
[0105] Use clade1-5 type VZV standard as the sample to be tested, human genome, herpes simplex virus 1, herpes simplex virus 2, cytomegalovirus, Epstein-Barr virus DNA as the control sample, globin probe as the internal standard, Verify the sensitivity and specificity of the kit of the present invention according to the following steps:
[0106] (1) adopt the specific primer shown in Table 3 of embodiment 1 to carry out multiplex PCR amplification to the genomic DNA of sample to be tested;
[0107] (2) The specific probes shown in Table 3 of Example 1 are respectively coupled with fluorescent microspheres of different numbers to prepare mixed microsphere hybridization solution;
[0108] (3) The hybridization product obtained after mixing the PCR product with the microsphere hybridization solution for hybridization reaction is loaded on the Luminex200 flow cytometry fluorescence...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com