Glycopeptide antibiotic derivatives
A technology of glycopeptide antibiotics and derivatives, applied in the direction of glycopeptide components, peptides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
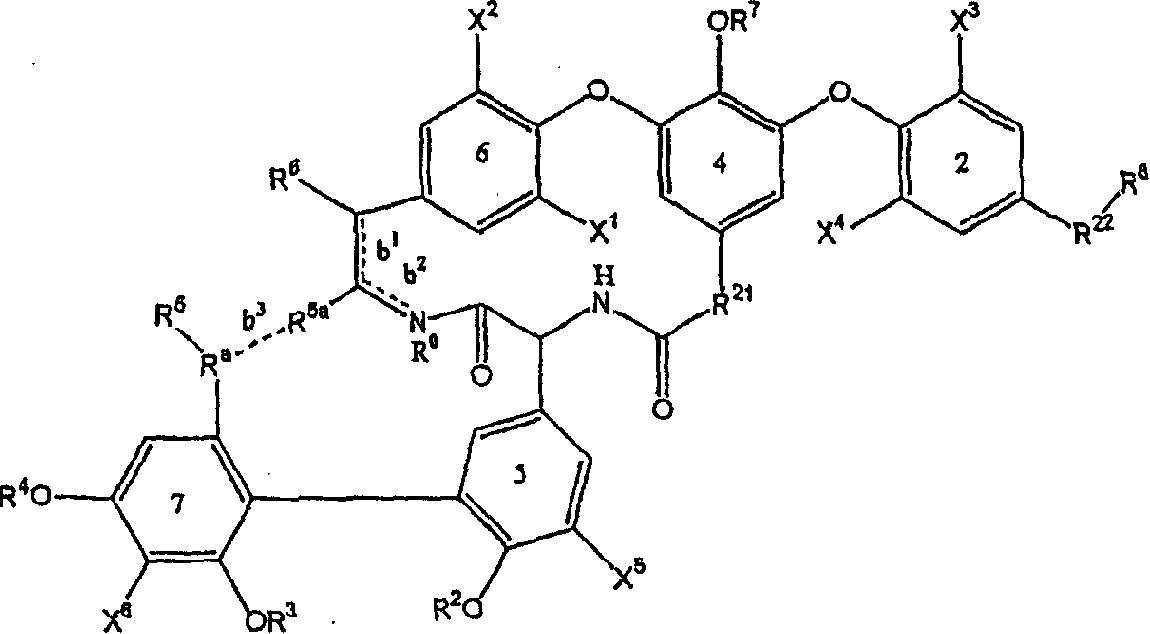
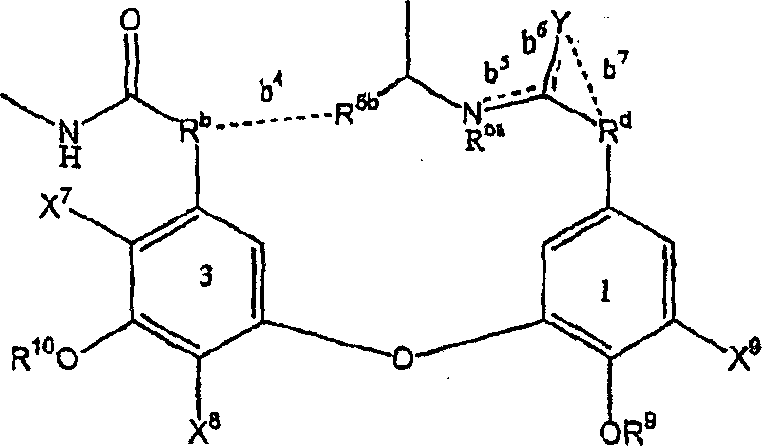
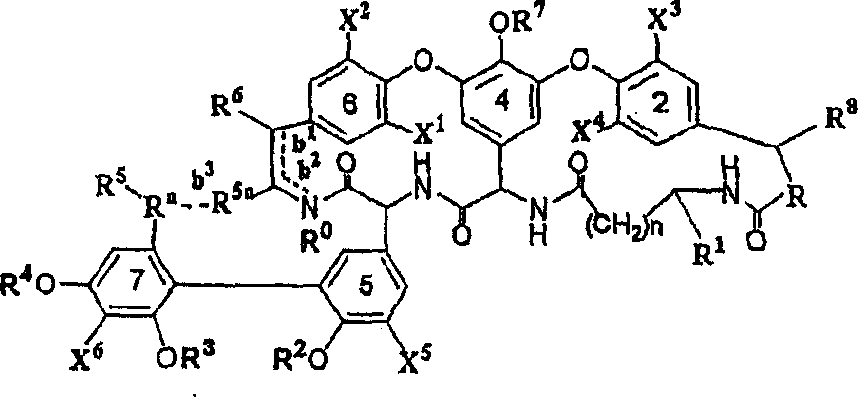
Image
Examples
Embodiment 1
[0246] Example 1: Tables 1-8 represent the structures of compounds prepared as examples and their respective codes
[0247] In this application, some compounds of the present invention are represented by the codes listed below.
[0248] Table 1. Vancomycin-like glycopeptides and their derivatives
[0249]
[0250]
[0251] Table 2. Teicoplanin-like glycopeptides and their derivatives
[0252]
[0253] the code
[0254] Table 3. N-deacylated-A40926 (DA40), demannosyl-N-deacylated A40926 (DMDA40) and their derivatives
[0255]
[0256] the code
[0257] Table 4. Vancomycin aglycones and their derivatives
[0258]
[0259] the code
[0260] Table 5. Teicoplanin aglycones and their derivatives
[0261]
[0262]
[0263] Table 6. Teicoplanin aglycone derivatives with amino acids 1 and 3 removed
[0264]
[0265] the code
[0266] Table 7. Teicoplanin Aglycon Derivatives Breaking the Bond Between Am...
Embodiment 2
[0273] Example 2: General Methods and Materials for Preparation of the Compounds
[0274] Glycopeptide antibiotics and their derivatives, especially the compounds of structural formula Z or I, II, III of the present invention can be prepared by a series of chemical reactions well known to those skilled in the art, which together constitute the preparation of said compounds and more Example method. The methods further described are merely illustrative and not intended to limit the scope of the invention.
[0275] The compounds of the present invention are conveniently prepared by one of the methods described below. The compounds shown in Tables 1-8 were prepared by the following preparation methods.
[0276] All reagents and solvents were purchased from Aldrich (Milwaukee), Fluka (Deisenhofen, Germany), Sigma (St. Louis, MO) and Merck (Darmstadt, Germany). This novel compound was obtained using methods described in the past for the synthesis of other glycopeptide derivativ...
Embodiment 3
[0304] Embodiment 3: measure antiviral (HIV, BVDV, HCV, HSV, VZV, CMV, FCV, SARS) and cell growth Methods of Inhibiting Activity
[0305] Anti-HIV activity assay
[0306] Inhibition of HIV-1 (IIIB, HE, HN) and HIV-2 (ROD, EHO, RF) cytopathic inhibition in CEM or C8166 or Molt4 / C8 cells was determined in 96-well plates in which Contains 3×10 5 CEM cells / ml, each ml was 100CCID 50 HIV-infected and containing appropriate dilutions of the compound to be tested. In control CO 2 The formation of CEM, C8166 or MOLT4 / C8 giant cells (syncytia) was detected microscopically after incubation at 37° C. for 4-5 days in a humidified atmosphere. EC 50 (50% effective concentration) is defined as the concentration of compound required to inhibit HIV-induced giant cell formation by 50%.
[0307] Cytostatic activity assay
[0308] All assays were performed in 96-well microtiter plates. Add 5-7.5 x 10 to each well 4 cells and add a certain amount of test compound. In humid controlled...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com