Medicinal preparation for preventing from pyloric helicobacterium inflammation
A technology of Helicobacter pylori and pharmaceutical preparations, which is applied in the field of immunity, can solve problems such as toxicity restrictions, and achieve the effect of convenient operation, simple process, and strong immune stimulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
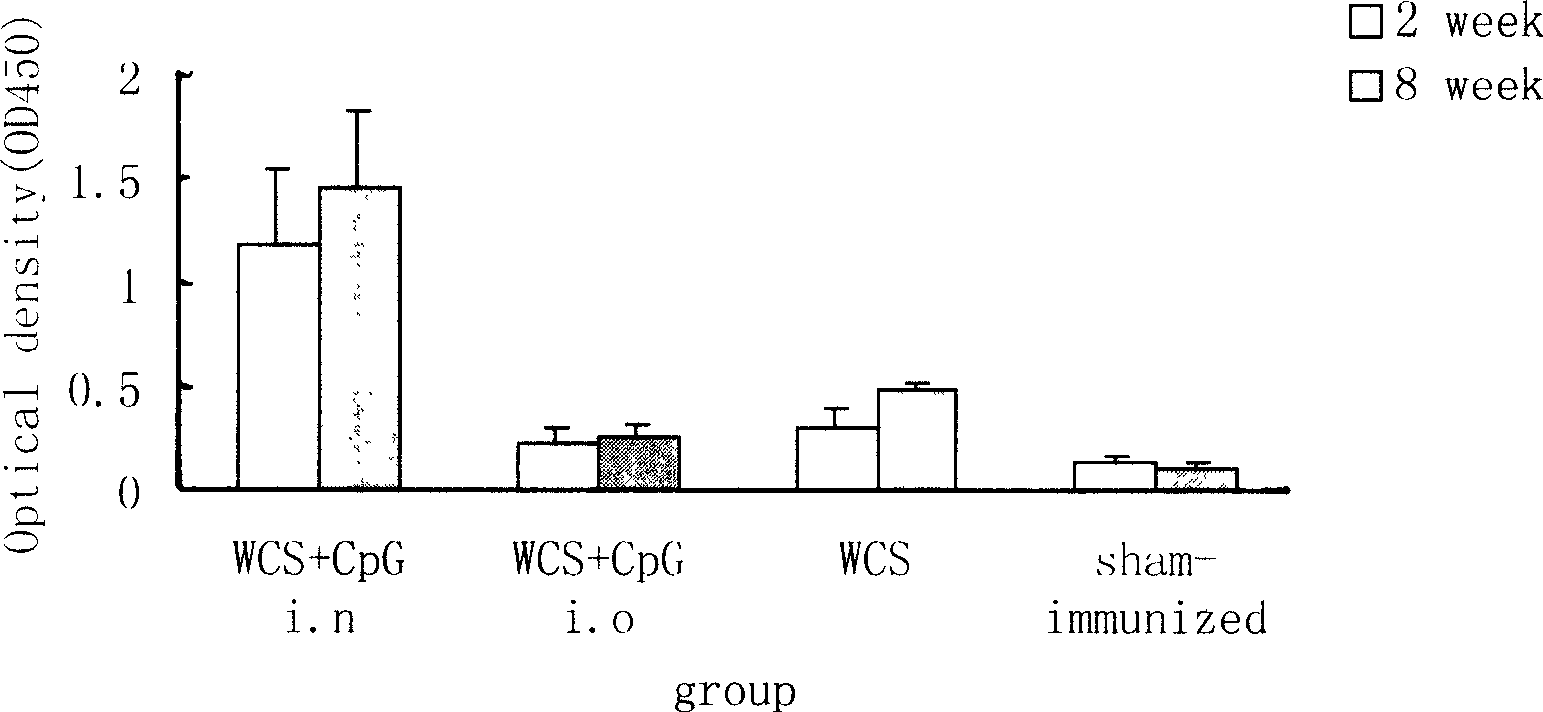
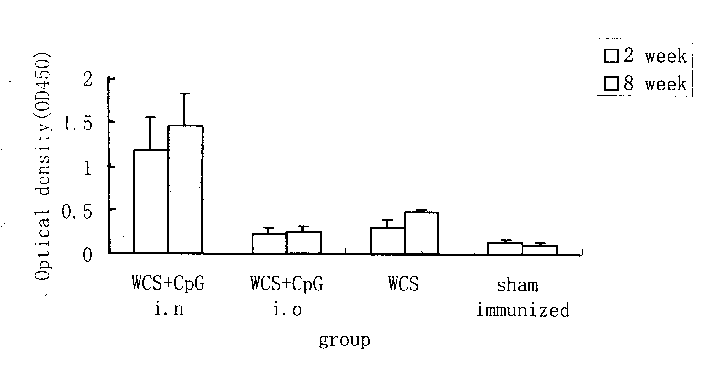
Image
Examples
Embodiment 1
[0011] Extraction of H.pylori genomic DNA:
[0012] H. pylori strain NCTC11637 (gifted by Professor Andren Lee, School of Microbiology and Immunology, University of New South Wales, Sydney, Australia) was grown on agar plates, cultured under microaerophilic conditions at 37°C for 48 hours, and the bacteria were collected in TE buffer at pH 8.0 In the method, after being treated with lysozyme, 10% SDS and 20mg / ml proteinase K, the whole genome DNA of Hp was extracted by phenol: chloroform extraction method, and kept at -20°C for later use.
Embodiment 2
[0014] Preparation of urease B subunit (UreaB) gene
[0015] Urease B subunit (UreaB) gene: The complete sequence of this gene was obtained from EMBL sequence No. M60398. Use the GCG gene analysis software system to analyze the restriction site, reading frame, and mRNA secondary structure of the target gene. The primers are designed as follows:
[0016] sense: 5'-AGGAAGATGCACGAAGACTGGGGCACC-3';
[0017] Antisense: 5'-GAAGATCTGGACGAAAATGCTAAATAGTTG-3'. PCR reaction conditions: heat denaturation at 94°C for 4min, 30 cycles of 30sec at 94°C, 30sec at 45°C and 1.5min at 72°C, and extension at 72°C for 10min. After the PCR products were identified by 1% Agarose electrophoresis, they were recovered by tapping the gel.
Embodiment 3
[0019] Preparation of outer membrane protein (OMP27) gene
[0020] The isolated and purified H. pylori genomic DNA was used as a template. Nucleic acid sequence design primers are as follows:
[0021] sense 5'-AAAAAAAGGTACCGAAGAGAGCGCGGCTTTTGTGGGA-3';
[0022] Antisense: 5'-AAAAAAAAGCTTTCATTAAAAATCCCTCAAGTAACTGAT-3'. PCR reaction conditions: heat denaturation at 94°C for 3 min, 30 cycles at 94°C for 30 sec, 50°C for 30 sec, and 72°C for 1 min, and extension at 72°C for 5 min. After the PCR products were identified by 1% Agarose electrophoresis, they were recovered by tapping the gel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com