Vaccine adjuvant comprising lipopeptide-inserted liposome as effective ingredient and use thereof
A vaccine adjuvant, liposome technology, applied in the directions of medical preparations containing active ingredients, liposome delivery, antibody medical ingredients, etc., can solve problems such as low efficacy, reduced incidence of herpes zoster, and no treatment methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0095] Hereinafter, the present invention will be described in detail by the following examples.
[0096] However, the following examples are only for illustrating the present invention, and the content of the present invention is not limited thereto.
Embodiment 1
[0097] Example 1 Preparation of recombinant varicella-zoster virus gE antigen
[0098] Construction of plasmid
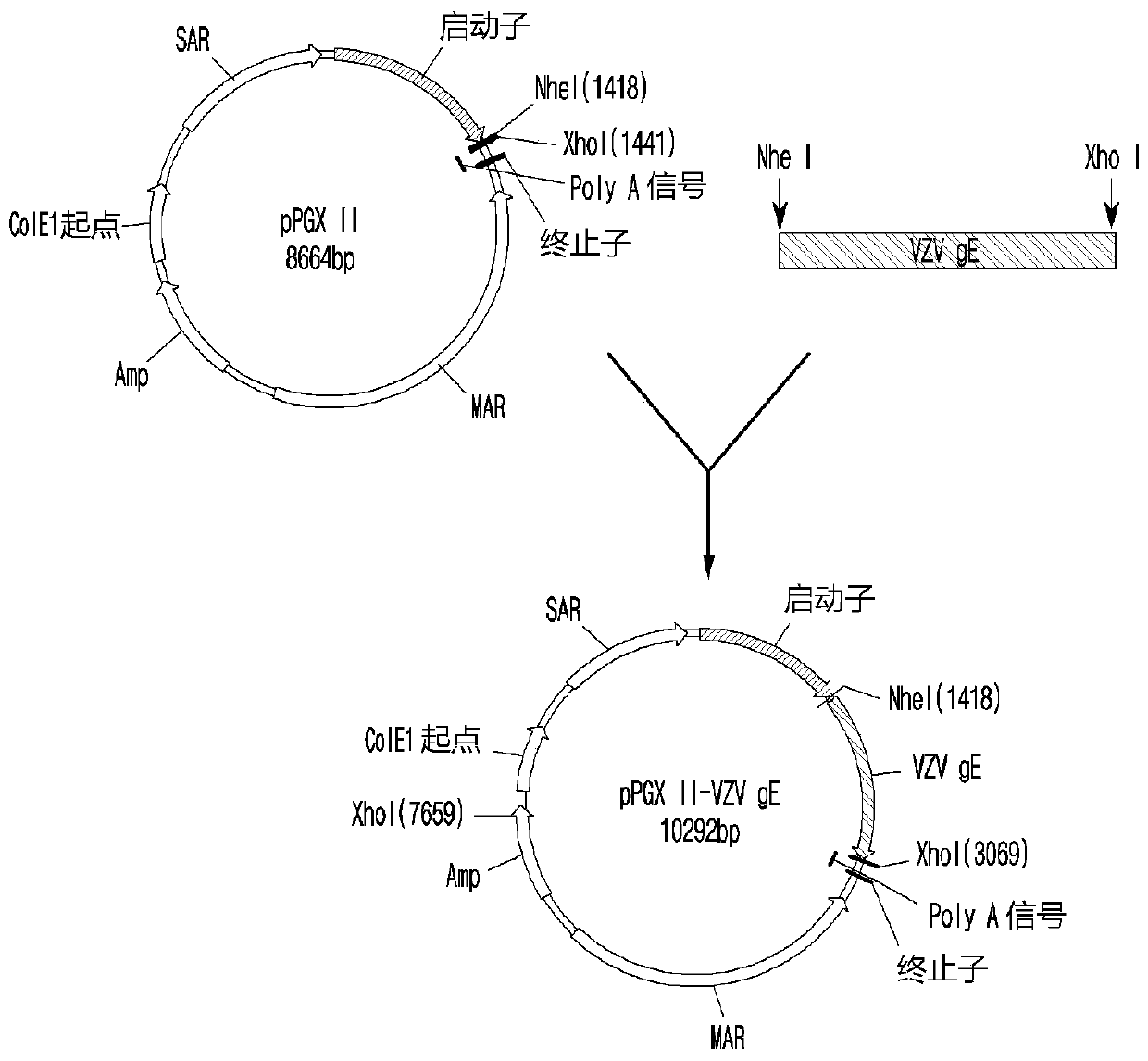
[0099] First, the gene (SEQ.ID.NO: 1) was synthesized to include a restriction enzyme recognition sequence (Nhe I site at 5' and Xho I site at 3') in the outer region of the gE (glycoprotein E) gene expression region of VZV. dots) and kozak sequences. At this point, a codon-optimized sequence for CHO cells, present with the C-terminal anchor domain removed from ORF68 (glycoprotein E) of the complete human herpesvirus type 3 (HHV-3) genome, was used as a template . The 1.6 kb gE gene of VZV represented by SEQ.ID.No: 1 was digested with Nhe I and Xho I restriction enzymes, and subcloned into pPGXII vector. As a result, pPGXII-VZV gE, the VZV gE expression plasmid ( figure 1 ).
[0100] Selection of cell lines
[0101] The DNA of the pPGXII-VZV gE plasmid prepared in Example was linearized with Ahd I restriction enzyme, and by electroporation, it was transfec...
Embodiment 2
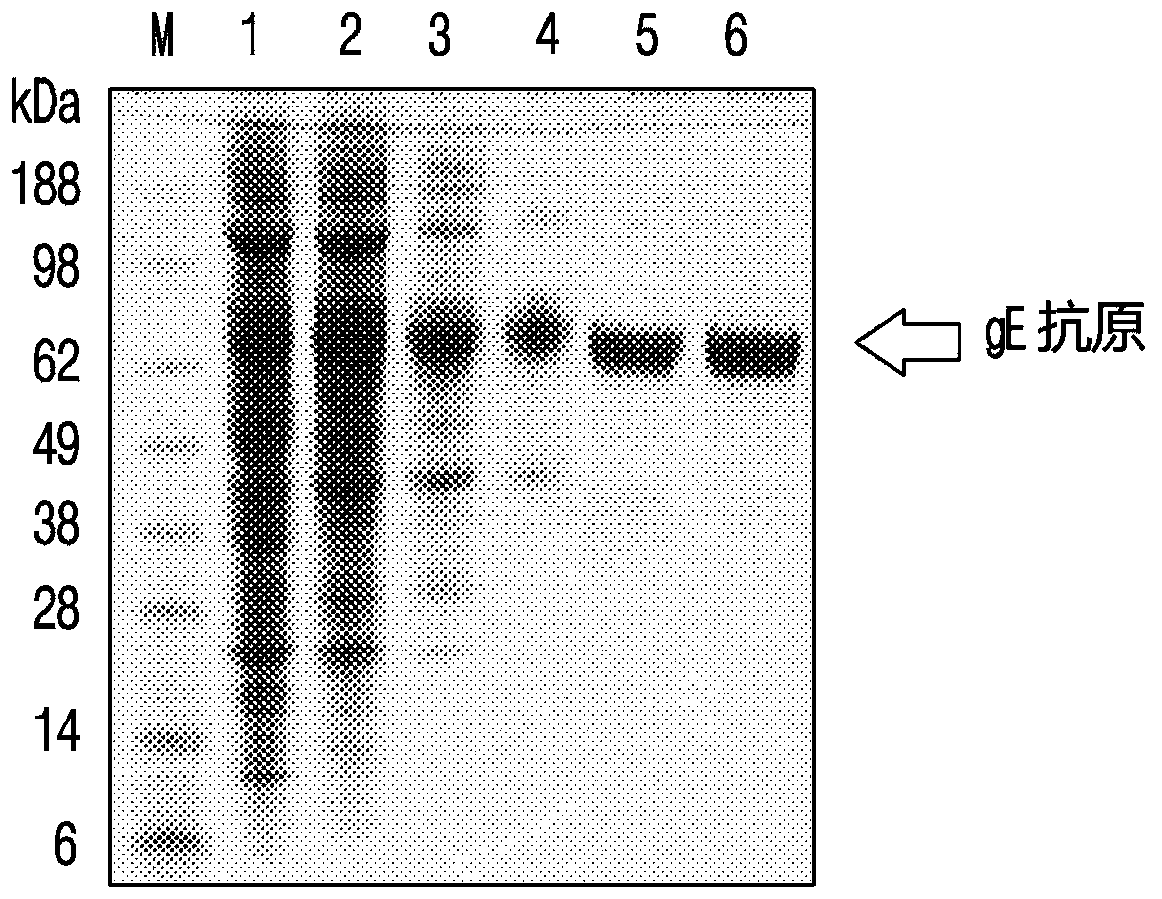
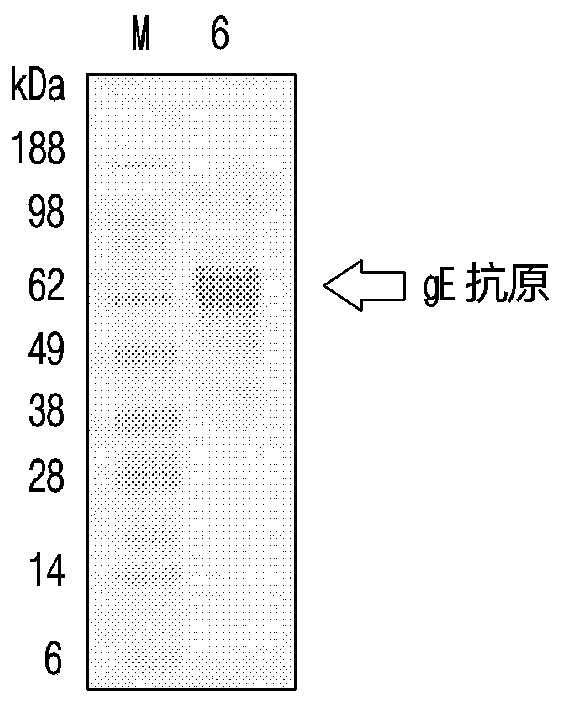
[0107] Embodiment 2 Comparison of the immunogenicity of the recombinant vaccine according to the dosage of lipopeptide and Poly(I:C)
[0108] Preparation and administration of test vaccine
[0109] First, to prepare DC-Chol:DOPE liposomes, DC-Chol and DOPE were dissolved in chloroform respectively, and then the organic solvent was vaporized with nitrogen gas while rotating the glass container, so that the ratio of DC-Chol and DOPE was 3:7. The mixed solution is evenly distributed on the bottom wall of the container. At this time, a thin film was formed on the bottom wall. The organic solvent remaining in the formed film was removed by storing in a vacuum desiccator for 1 hour. Distilled water was added to the completely dried lipid film and then fully hydrated using an ultrasonic bath for 10 min. In preparing multilamellar vesicle (MLV) suspensions, a 2X buffer solution (pH 7.0) containing 300 mM NaCl in 20 mM sodium phosphate was added in the same amount as distilled wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com