Method for preparing antigen tablet for detecting varicella-zoster virus antibody
An antibody detection, chickenpox virus technology, applied in the direction of virus/phage, biochemical equipment and methods, biological testing, etc., to achieve high qualification rate, solve the problem of complex production process, simplify the effect of preparation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
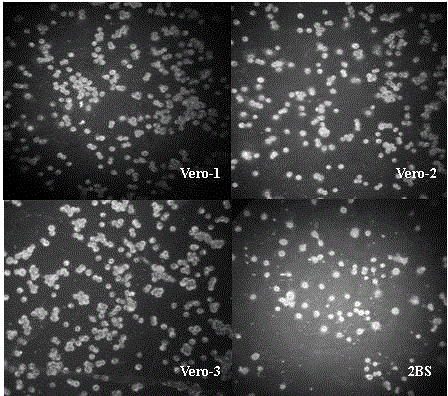
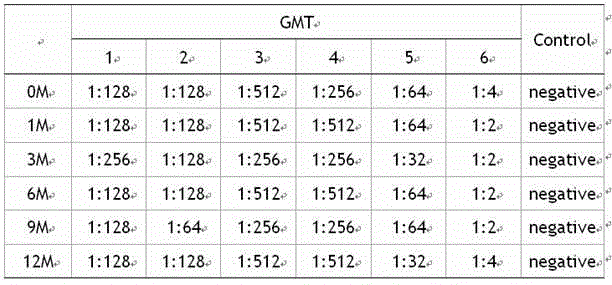
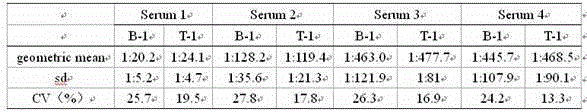
[0024] Select Vero cells that grow to a monolayer with a density of about 2.5—3.5×10 5 / ml; varicella-zoster virus strain VZV 84-7 virus titer is 4.9lgPFU / ml; Cells are infected with a multiplicity of infection of 0.01; virus infection solution is MEM containing 1% glutamine, 2% newborn bovine serum, with 5.6 %NaHCO 3 Adjust the pH value to 7.5±0.1; place the infected cells in a constant temperature incubator at 35°C and culture until the peak of virus replication; digest with 0.25% trypsin, and count the cells; add 1% glutamine and 5% newborn bovine serum to a pH value of 7.4 ±0.1 MEM culture solution, adjust the cell concentration to 1.2~1.8×10 5 / ml to prepare cell suspension; drop the cell suspension into 12-well glass slides, 20~25μl per well, put the slides in a humid box, and put them at 35°C and 5% CO 2 After incubating in the incubator for 30 minutes, rinse with PBS once, dry naturally, and directly fix with cold acetone at room temperature for 15 minutes, take it o...
Embodiment 2
[0026] Select Vero cells that grow to a monolayer with a density of about 2.5—3.5×10 5 / ml; varicella-zoster virus strain VZV 84-7 virus titer is 5.2lgPFU / ml; Cells are infected with a multiplicity of infection of 0.01; the virus infection solution is MEM containing 1% glutamine, 2% newborn bovine serum, with 5.6 %NaHCO 3 Adjust the pH value to 7.5±0.1; place the infected cells in a constant temperature incubator at 35°C and culture until the peak of virus replication; digest with 0.25% trypsin, and count the cells; add 1% glutamine and 5% newborn bovine serum to a pH value of 7.4 ±0.1 MEM culture solution, adjust the cell concentration to 1.2~1.8×10 5 / ml to prepare cell suspension; drop the cell suspension into 12-well glass slides, 20~25μl per well, put the slides in a humid box, and put them at 35°C and 5% CO 2 After incubating in the incubator for 30 minutes, rinse with PBS once, dry naturally, and directly fix with cold acetone at room temperature for 15 minutes, take ...
Embodiment 3
[0028] Select Vero cells that grow to a monolayer with a density of about 2.5—3.5×10 5 / ml; varicella-zoster virus strain VZV 84-7 virus titer is 4.9lgPFU / ml; Cells are infected with a multiplicity of infection of 0.05; the virus infection solution is MEM containing 1% glutamine, 2% newborn bovine serum, with 5.6 %NaHCO 3 Adjust the pH value to 7.5±0.1; place the infected cells in a constant temperature incubator at 35°C and culture until the peak of virus replication; digest with 0.25% trypsin, and count the cells; add 1% glutamine and 5% newborn bovine serum to a pH value of 7.4 ±0.1 MEM culture solution, adjust the cell concentration to 1.2~1.8×10 5 / ml to prepare cell suspension; drop the cell suspension into 12-well glass slides, 20~25μl per well, put the slides in a humid box, and put them at 35°C and 5% CO 2 After incubating in the incubator for 30 minutes, rinse with PBS once, dry naturally, and directly fix with cold acetone at room temperature for 15 minutes, take ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com