Varicella-zoster virus r-gE fusion protein, recombinant varicella-zoster vaccine and preparation method and application of varicella-zoster virus r-gE fusion protein and recombinant varicella-zoster vaccine
A herpes zoster virus and fusion protein technology, applied in the field of biomedicine, can solve problems such as weak cellular immunity, achieve simple preparation process, convenient use, prevent or improve the effect of neuralgia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1 r-gE fusion protein eukaryotic expression plasmid construction
[0058] The antigen used in a recombinant varicella-zoster vaccine is in the form of r-gE fusion protein, including VZV gE extracellular sequence and ORF7 protein fragment. VZV gE selects the N-segment extracellular sequence of Dumas strain (GeneBank No.: NP_040190.1), and ORF7 sequence (GeneBank No.: AXF42671.1) selects the N-segment amino acid sequence. The amino acid sequence of the r-gE fusion protein is shown in SEQ ID NO.1; the theoretical molecular weight of the protein is about 66kD (the protein undergoes intracellular modification after expression, and the actual molecular weight may increase).
[0059] The r-gE fusion protein gene was synthesized by Nanjing GenScript Co., Ltd., and the delivered product was a pUC57 plasmid vector. HindIII and EcoR1 restriction sites were introduced at both ends of the expression framework, and the target gene was codon-optimized according to the CHO c...
Embodiment 2
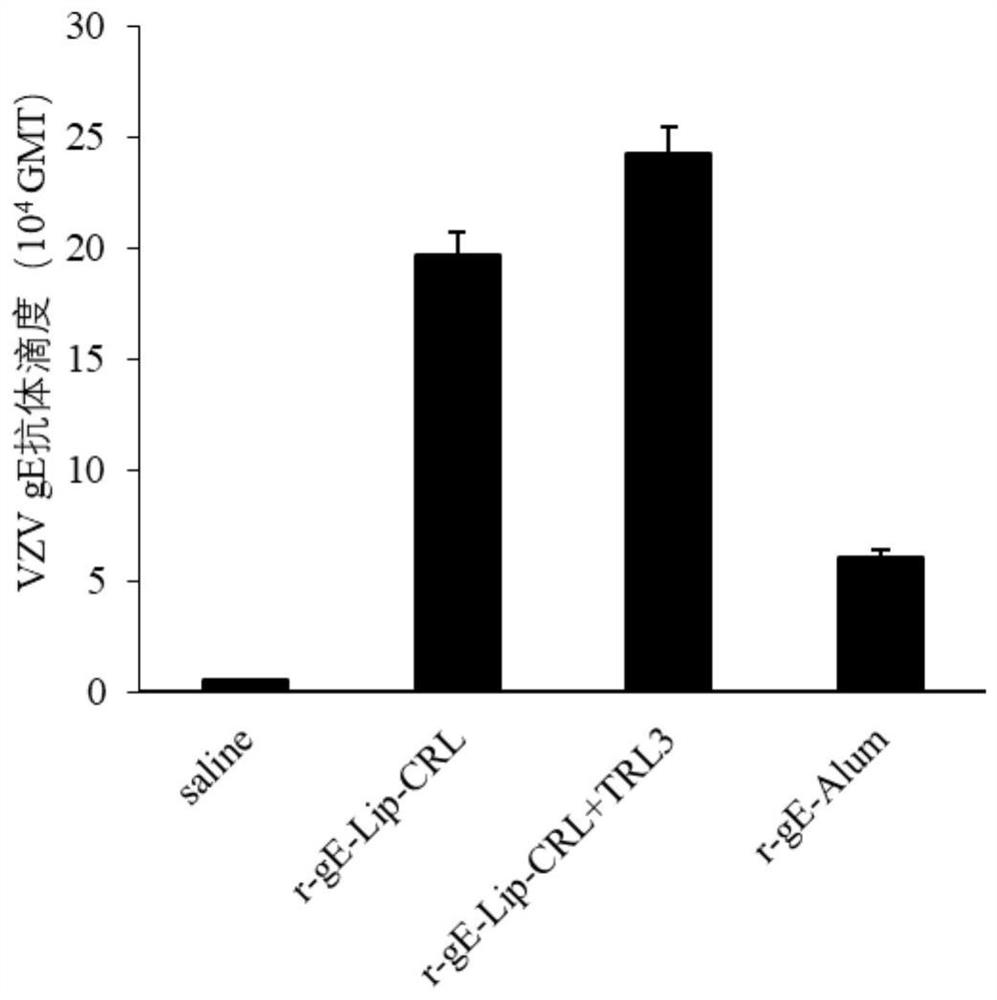
[0061] Embodiment 2 liposome adjuvant compatible varicella-zoster vaccine
[0062] The vaccine is prepared by r-gE fusion protein and liposome containing agonist, and the specific preparation method is as follows.
[0063] 2.1. Preparation of antigenic protein
[0064] Referring to the method recorded in the "Molecular Cloning Experiment Guide", the specific preparation method of the antigen r-gE fusion protein is as follows:
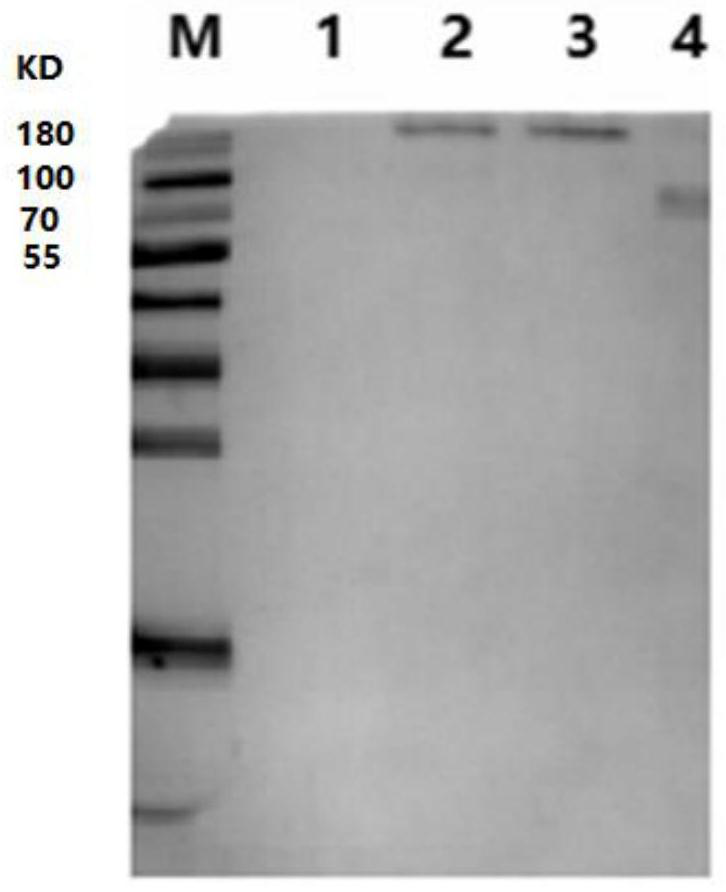
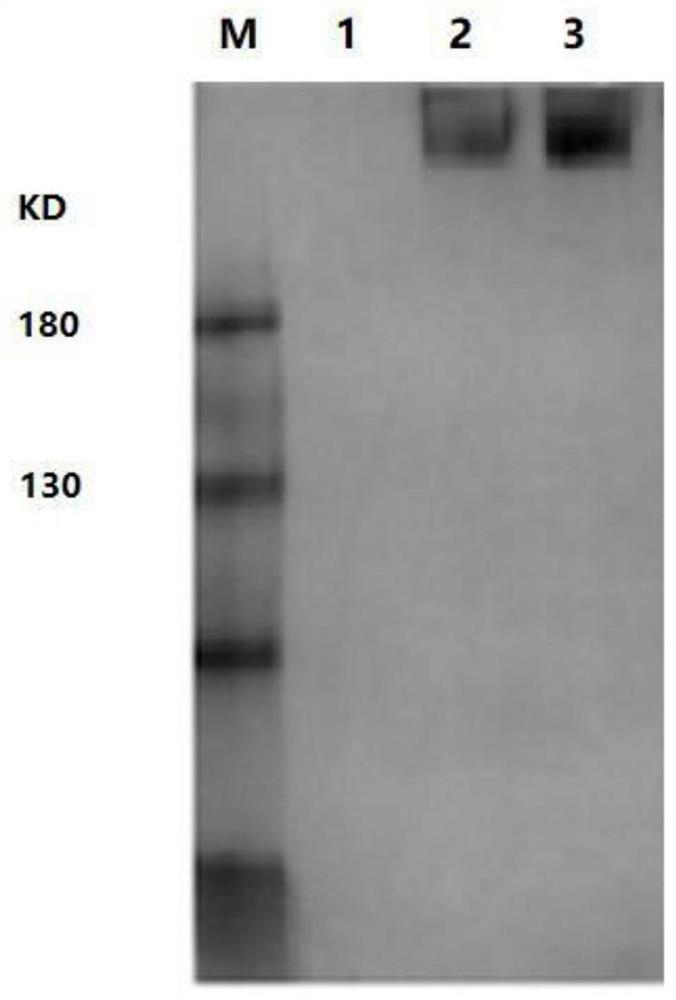
[0065] S1. Transfection of the recombinant plasmid: after linearizing the pEE12.4-r-gE plasmid constructed by the method described in Example 1, transfect it into CHO cells using lip2000 liposomes (refer to the lip2000 liposome transfection instructions); After 48 hours of transfection, take the cell supernatant and use Western Blot to detect the expression of r-gE fusion protein; after 48 hours of transfection, take the cell supernatant and use Western Blot to detect the expression of r-gE fusion protein; , using gE protein mouse monoclonal antibody ...
Embodiment 3
[0082] Embodiment 3 recombinant adenovirus vector varicella-zoster vaccine
[0083] Recombinant adenoviral vector varicella-zoster vaccine (recombinant adenoviral vector vaccine preparation) is recombined from r-gE fusion protein and adenoviral vector, denoted as vaccine 4 (r-gE-Ad), or r-gE fusion The protein is recombined with an adenovirus vector expressing a TLR receptor agonist, and is designated as vaccine 5 (r-gE-R-Ad).
[0084] The specific preparation method is as follows:
[0085] 3.1. Preparation of herpes zoster recombinant adenovirus vector virus
[0086] Experimental materials and sources: The gene sequence of the r-gE fusion protein was synthesized by a commercial company, and the amino acid sequence of the protein is shown in SEQ ID NO.1; DNA Polymerase was purchased from Novozyme, and LR recombinase was purchased from Invitrogen.
[0087] Preparation method: The method described in Example 1 was used to construct the r-gE fusion protein gene expression plasm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Theoretical molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com