Varicella zoster virus-virus like particles (VLPS) and antigens
A technology of varicella zoster and VZV-VLP, which is applied in the direction of viruses, antiviral agents, viruses/phages, etc., and can solve problems such as restricting the application of vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Cells, Viruses, and Constructs
[0101] Spodoptera frugiperda Sf9 insect cells (ATCC CRL-1711 ) were maintained at 28°C as suspension cultures in HyQ-SFX insect serum-free medium (HyClone, Logan, UT). The Bac-to-Bac (bacteria to bacteria) baculovirus expression system (Invitrogen, Carlsbad, CA) was used with the pFastBacl transfer vector in E. coli DH10Bac cells to generate recombinant baculovirus vectors expressing influenza genes.
[0102] The VZV gene is based on the GenBank sequence NC_001348. The gene encoding the protein (see below) was codon optimized for high level expression in Sf9 cells and synthesized at GeneArt (Regensburg, Germany). The synthetic gene was transferred into pFastBac1, downstream of the AcMNPV polyhedrin promoter, as previously described in detail for influenza virus (Pushko et al., 2005.).
[0103]Recombinant baculoviruses were generated by site-specific homologous recombination after transformation of a transfer plasmid containing the VZV ...
Embodiment 2
[0190] Expression of VZV gE protein alone forms VLPs
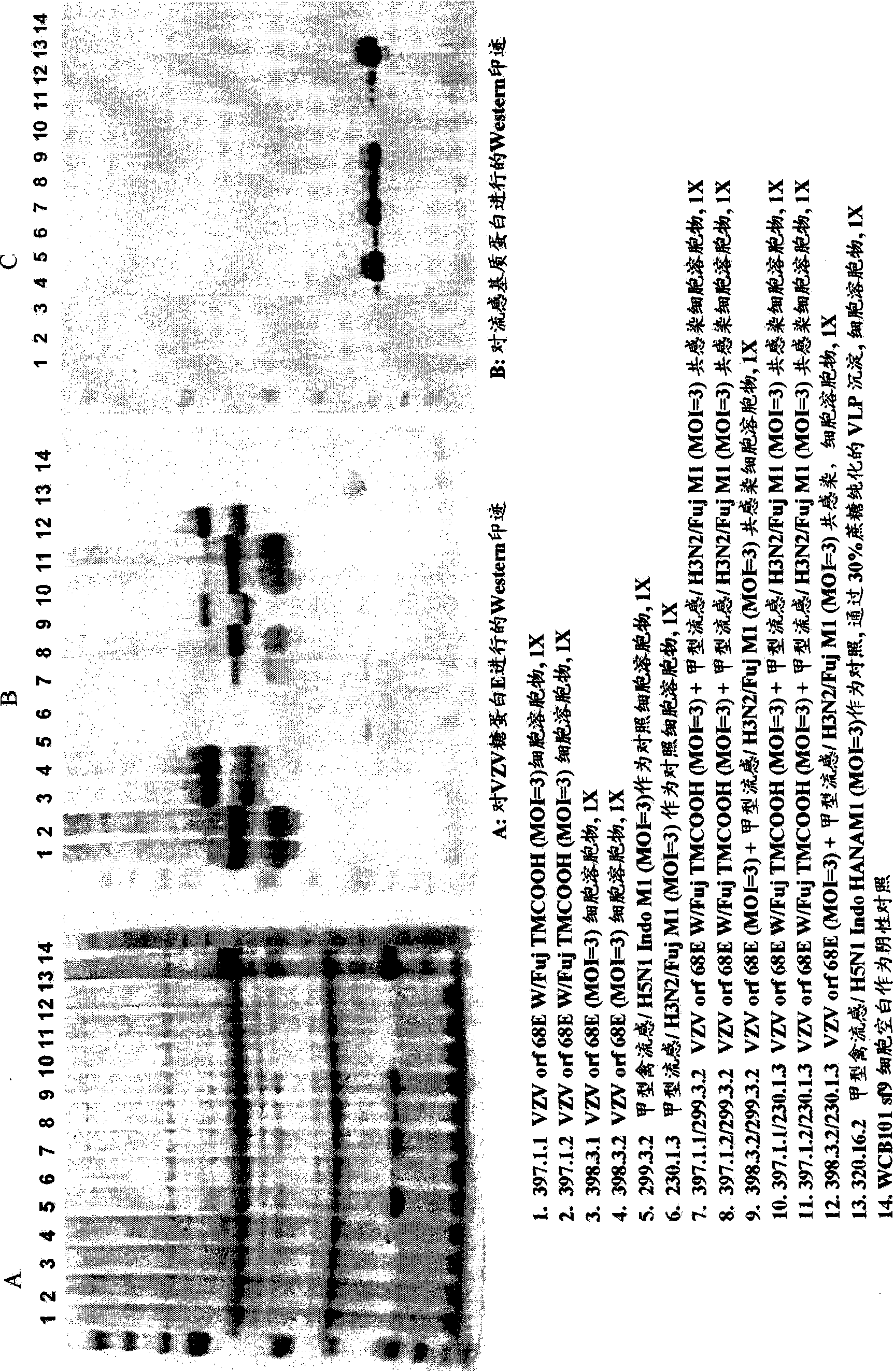
[0191] Baculovirus constructs containing only VZV gE were expressed in SF9 cells and analyzed following the protocol above. Particles were purified from Sf9 cells infected with the BV-VZV gE vector via a 20%-60% sucrose density gradient step using the method described above. Gels and Western blots confirmed recovery of VZV gE in the particle fraction of the sucrose gradient. Run samples on SDS gels ( figure 1 A), and Western blot of the isolated supernatant probed for VZV gE ( figure 1 B) or influenza matrix protein ( figure 1 C). Such as figure 1 As shown in lanes 2 and 3 of B, expression of VZV gE protein alone results in the formation of VZV-VLPs.
[0192] In addition, analysis of gradient purified particles by size fractionation on a Sephacryl S-400 gel permeation chromatography column was performed. Most gE proteins are larger than 6,000 kDa, which is consistent with this being a VLP ( figure 2 ). Thus, expr...
Embodiment 3
[0194] Express for IE62
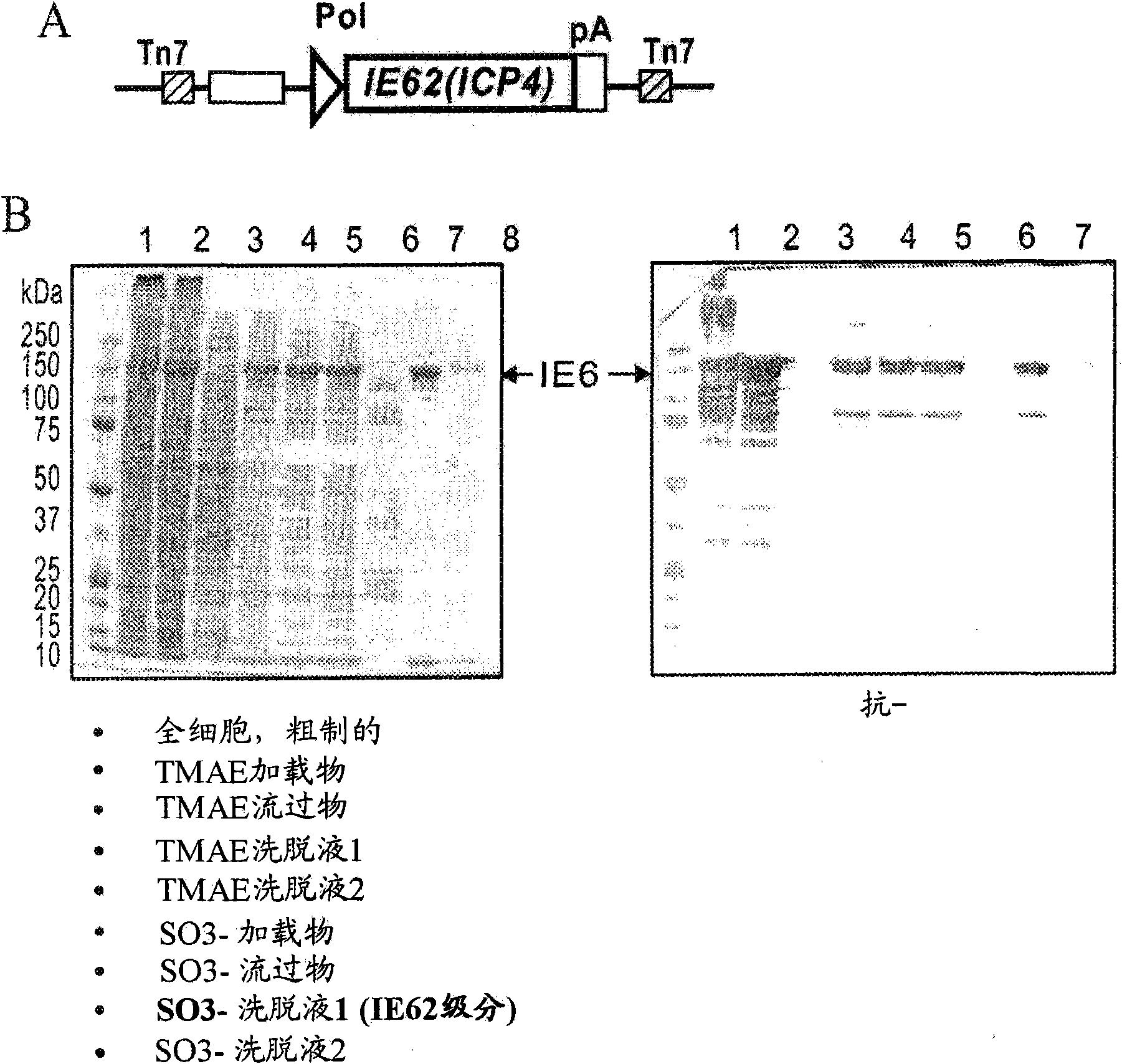
[0195] IE62 is the major VZV interlayer protein. Immunization induces specific antibodies and cell-mediated immunity (CMI), which protects guinea pigs upon challenge with VZV. Described is the full-length VZV IE62 gene cloned into a baculovirus expression vector ( image 3 A) Recombinant IE62 produced in Sf9 insect cells, and the native method used to extract and purify intracellular IE62.
[0196] method. Baculoviruses were engineered to express the full-length, codon-optimized IE62 gene from the Oka strain of VZV. The gene was synthesized (GeneArt, Germany) and cloned into the pFastBac1 vector under the control of the baculovirus polyhedrin promoter (Invitrogen). This gene was transferred to the AcMNPV bacmid (Invitrogen) and the bacmid DNA was used to transfect Sf9 insect cells. The resulting recombinant baculoviruses were plaque purified and virus stocks were prepared in Sf9 cells.
[0197]Orf IE62 ICP4 full length. ATGGACACCCCCCCCATGCAGCGT...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com