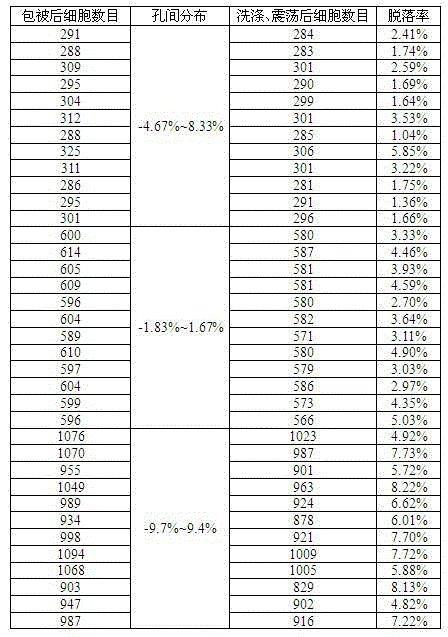
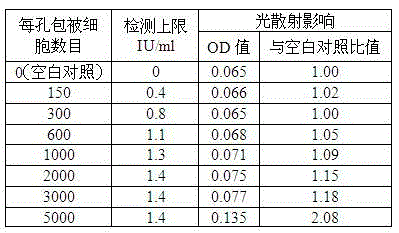
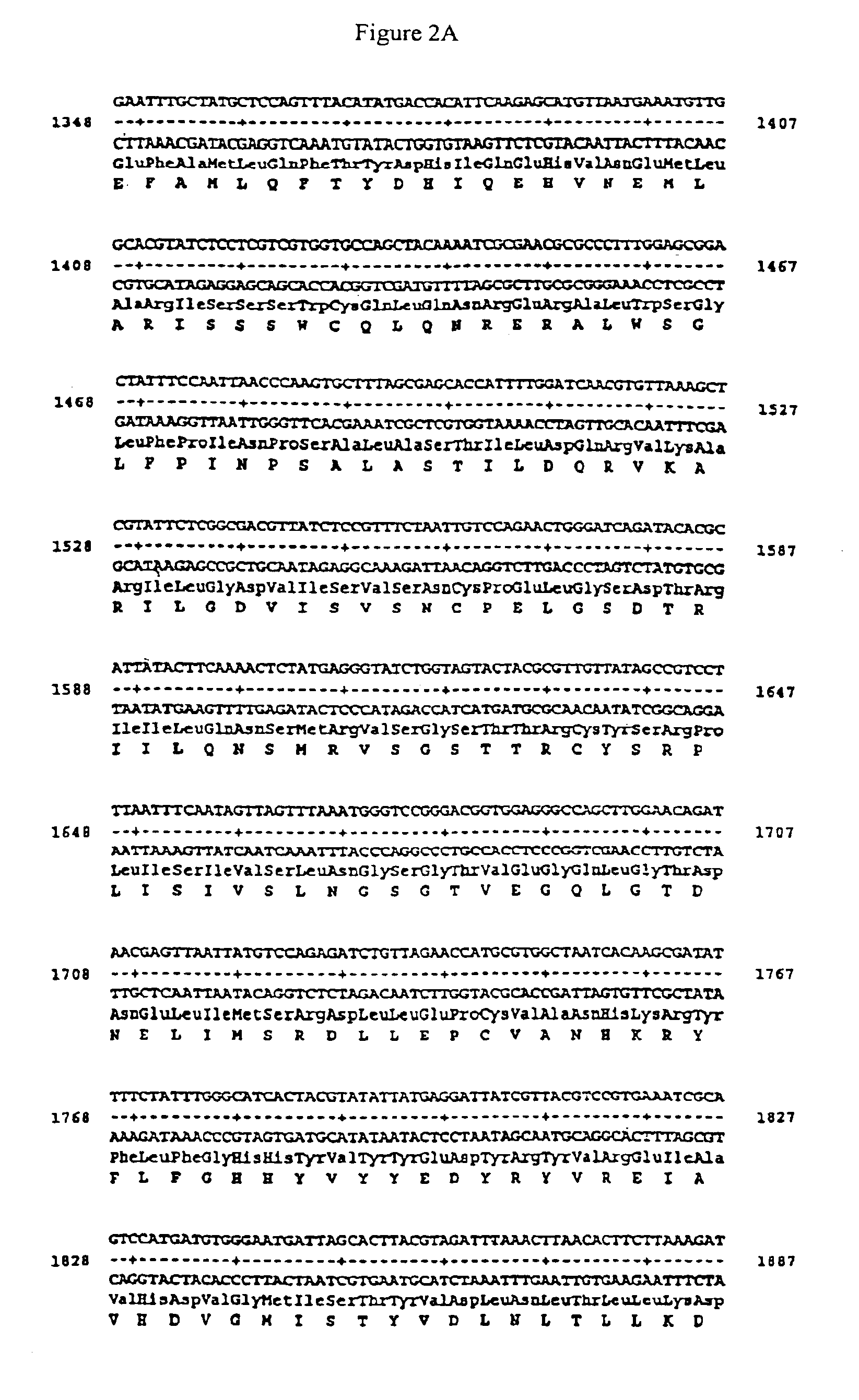
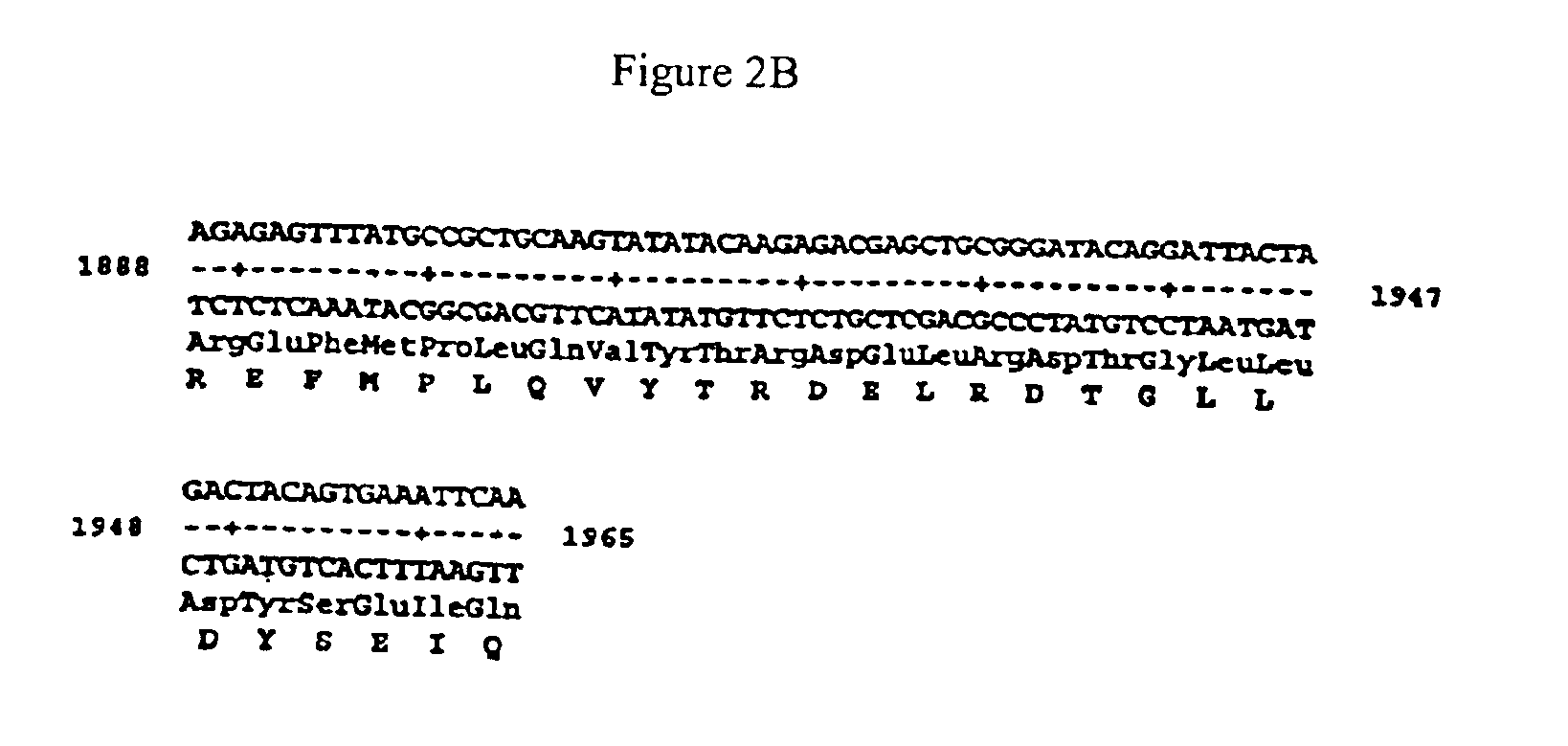
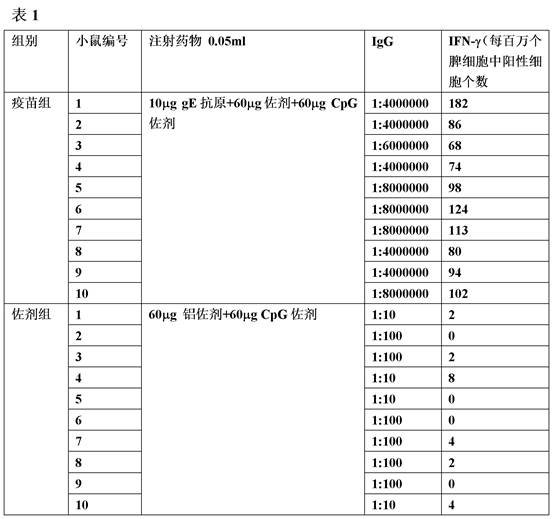
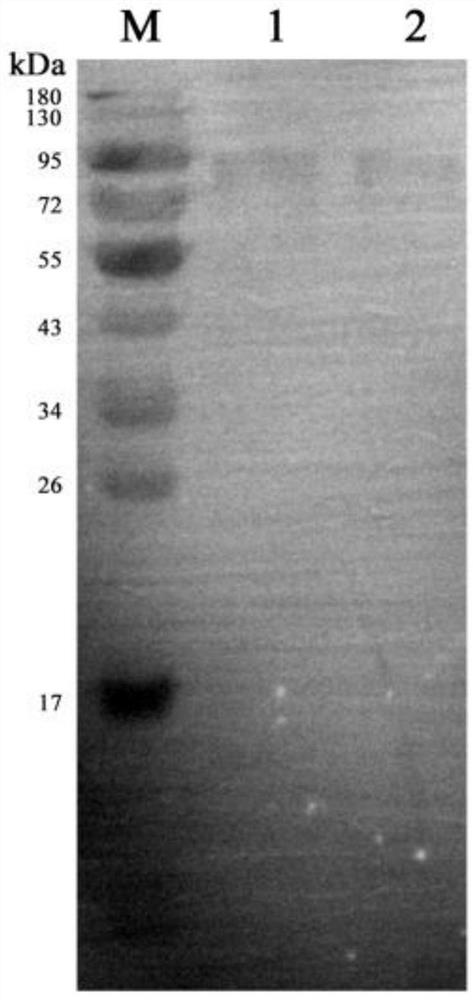
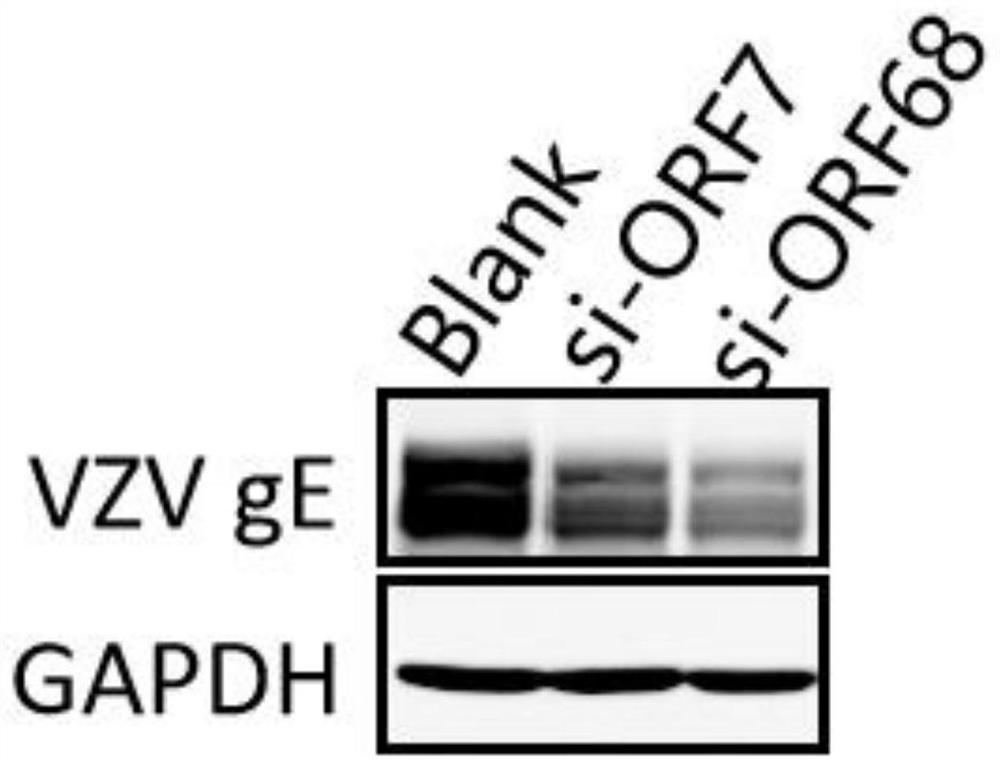
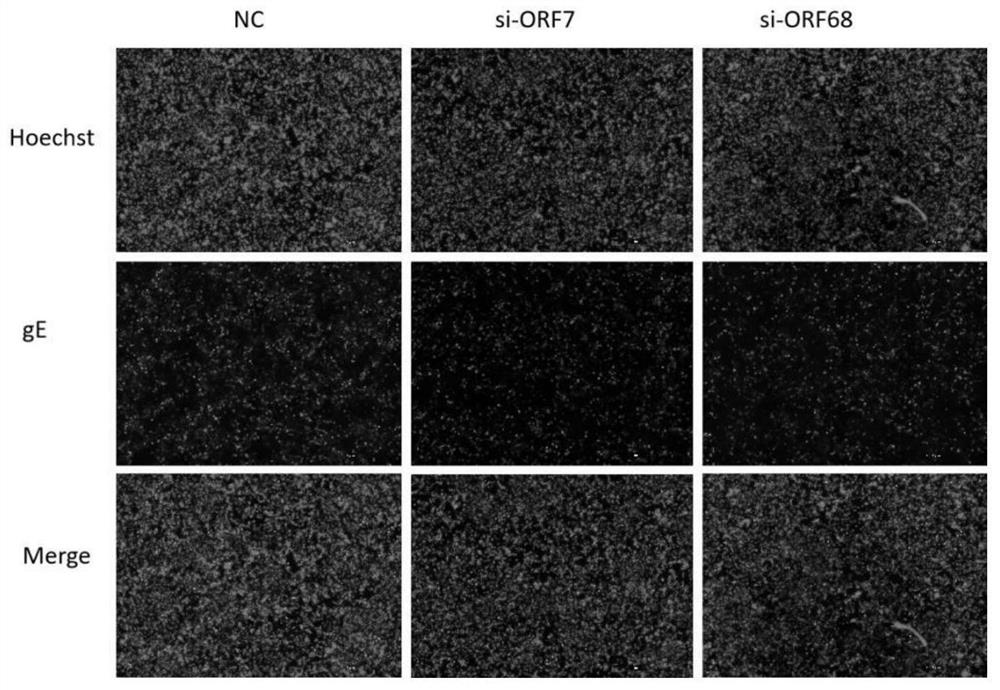
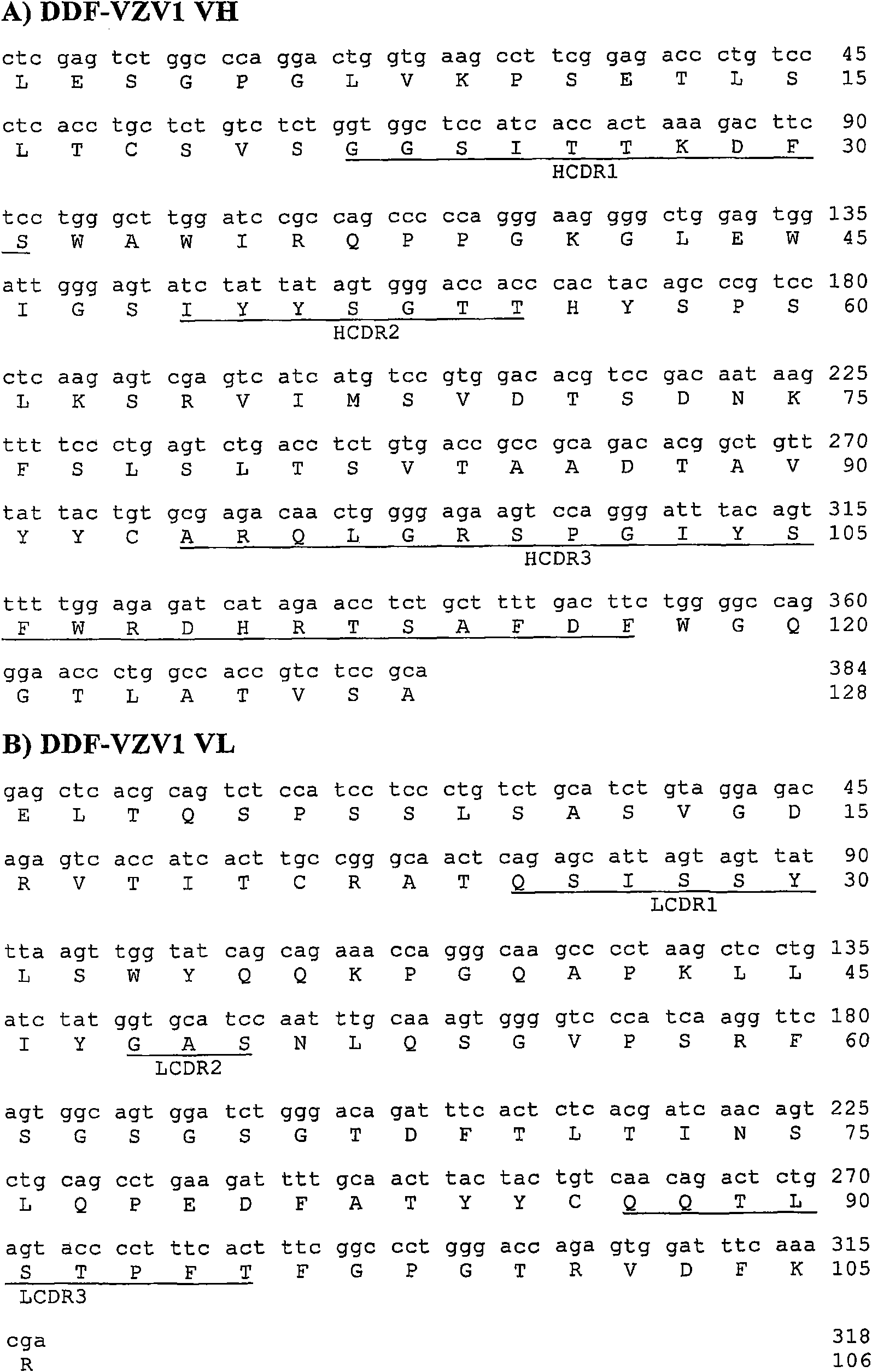Patents
Literature
56 results about "VZV - Varicella-zoster virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Synthetic peptide, inhibitor to DNA viruses
The present invention relates to the identification of the active domain of Herpoxin, a DNA virus-inhibiting-protein which was isolated from cobra venom in U.S. Pat. No. 5,648,339 and has a molecular weight of 13.5 kDa We have isolated a fragment of Herpoxin which contains the active domain and which we have named Herp. Herp mimics the activity of Herpoxin in inhibiting the replication of DNA viruses. A synthetic version of the active fragment was produced having the amino acid sequence Asn-Leu-Tyr-Gln-Phe-Lys-Asn-Met-Ile-Gln. The synthetic version of Herp consisting of ten amino acids inhibits the replication of DNA viruses such as herpes viruses types 1 and 2, cytomegalovirus and varicella zoster virus as well as Tubercle bacilli.
Owner:LIPPS BINIE V +1
Varicella zoster virus-virus like particles (VLPS) and antigens
The present invention discloses novel Varicella Zoster Virus (VZV) virus-like particles (VLPs) comprising glycoprotein E of VZV. The invention also discloses vaccine formulations of the VZV-VLPs and methods of inducing an immune response in subjects.
Owner:NOVAVAX
Application of polycyclic polyketides in preparation of anti-HV (herpes virus) drug
The invention discloses an application of polycyclic polyketides in preparation of an anti-HV (herpes virus) drug. It is found that the polyketides can inhibit diseases caused by infection of four HVs including HSV-1 (herpes simplex virus-1), HSV-2 (herpes simplex virus-2), VZV (varicella zoster virus) and CMV (cytomegalo virus). The compounds show equivalent activity but have different acting mechanisms as compared with commercial drugs such as acyclovir and can overcome drug resistance of existing commercial drugs. Therefore, the compounds have good application prospects in treatment of related diseases caused by infection of HVs including HSV-1, HSV-2, VZV and CMV.
Owner:JINAN UNIVERSITY
Recombinant varicella zoster virus vaccine
ActiveCN112870344AHigh molecular weightImproving immunogenicityPeptide/protein ingredientsAntibody mimetics/scaffoldsChickenpoxImmunogenicity
The invention discloses a recombinant varicella zoster virus vaccine, which comprises an amino acid sequence of a recombinant glycoprotein gE extracellular region of a live attenuated VZV strain (OKA strain) gene and a fusion protein formed by a human immunoglobulin Fc segment, and further comprises preparation and an application of the fusion protein, and a corresponding recombinant gene, an eukaryotic expression vector and the like. The fusion protein provided by the invention has good immunogenicity, and can induce generation of a high-level serum neutralizing antibody.
Owner:BEIJING LUZHU BIOTECH +1
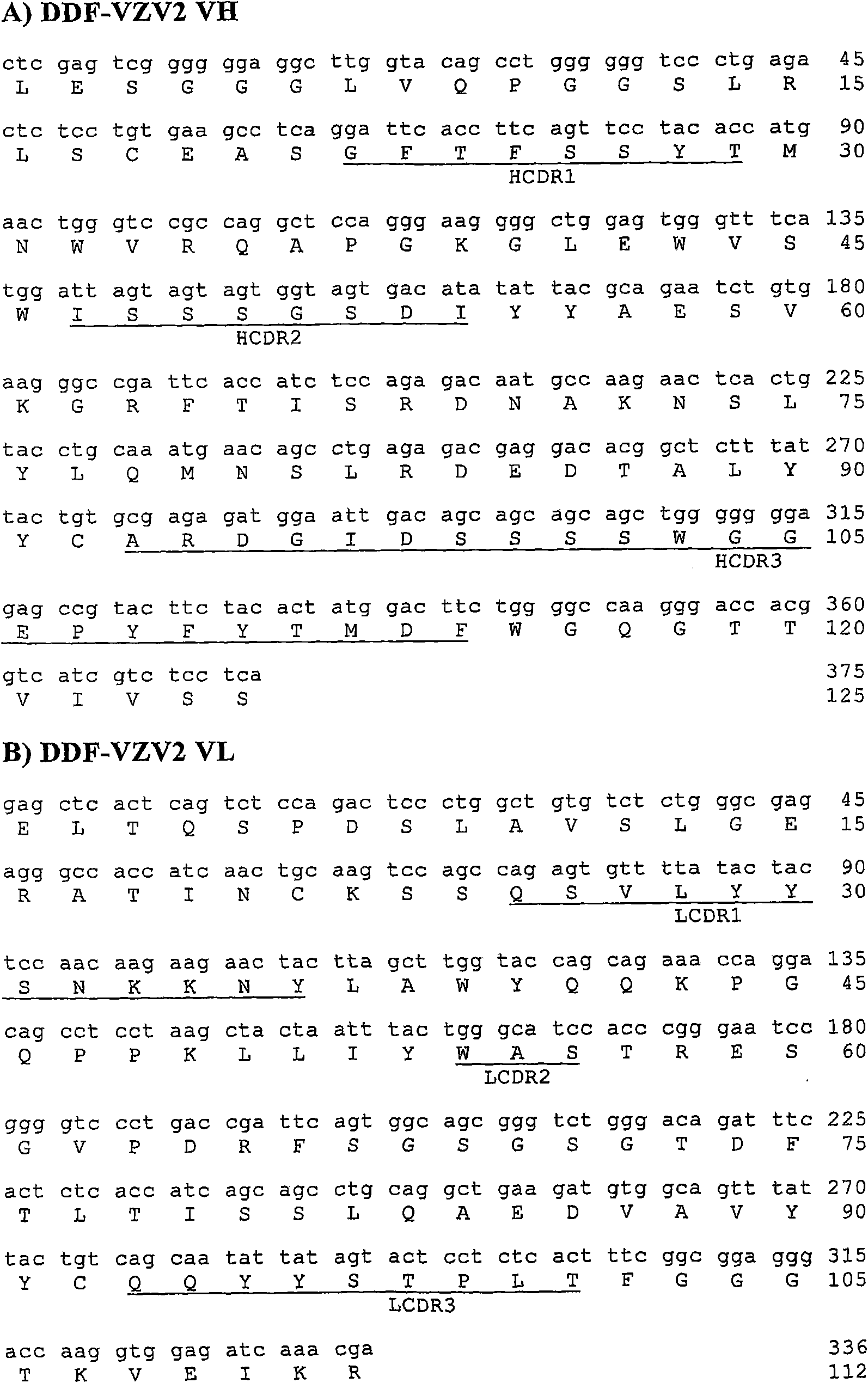
Antibodies specific for varicella zoster virus
InactiveCN101663318AVirus peptidesImmunoglobulins against virusesTherapeutic treatmentVaricella zoster virus
The present invention provides novel antibody sequences that bind Varicella Zoster Virus (VZV) and neutralize VZV infection. The novel sequences can be used for the medical management of VZV infection, in particular for detecting the virus or for preparing pharmaceutical compositions to be used in the prophylactic or therapeutic treatment of VZV infection.
Owner:RIBOVAX BIOTECHNOLOGIES SA
Kit for genotyping VZV, production method of kit and application of kit
InactiveCN105132584ANo cross reactionStrong specificityMicrobiological testing/measurementMicroorganism based processesHuman DNA sequencingChemical structure
The invention provides a kit for genotyping VZV (Varicella-Zoster Viruses). The kit is characterized by comprising a nucleotide sequence shown as the Table 3 in the specification, and specific primers and specific probes corresponding to clade1-5 type VZV of chemical structures. The kit has the function of detecting various kinds of VZV DNA (Deoxyribonucleic Acid); the detection sensitivity is 10<2> copies / reaction; no cross reaction with human genome, herpes simplex viruses type I / type II, cytomegaloviruses and EB viruses exists; and the kit is applicable to the virus gene diagnosis of clinical VZV infected persons, and can also be used for the epidemiology survey of different Clade types of VZV.
Owner:CHENGDU MILITARY GENERAL HOSPITAL OF PLA
Methods and compositions for detecting CNS viruses
The present invention generally relates to a molecular test of enterovirus, herpes simplex virus-1 and -2, and / or Varicella-Zoster virus, in order to identify patients with a viral infection, in particular a viral infection of the central nervous system. Accordingly methods and compositions are disclosed to determine the presence or absence of a viral pathogen in a biological sample comprising, wherein the target nucleic acids comprise the 5′ UTR of the enterovirus genome, UL29 of herpes simplex virus and gene 36 of Varicella-Zoster virus.
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
Varicella-zoster virus gE antigen and application thereof in detection of anti-varicella-zoster virus antibody
The invention discloses a varicella-zoster virus gE glycoprotein antigen capable of detecting an anti-varicella-zoster virus antibody, particularly an anti-varicella-zoster virus neutralizing antibody, and also discloses application of the antigen in the preparation of a detection reagent for detecting the anti-varicella-zoster virus neutralizing antibody and a corresponding detection reagent kit.
Owner:北京市华信行生物科技有限公司
Conditionally replication deficient herpes virus and use thereof in vaccines
ActiveUS20160008458A1Animal cellsAntibody mimetics/scaffoldsHerpesvirus infectionSimian varicella virus
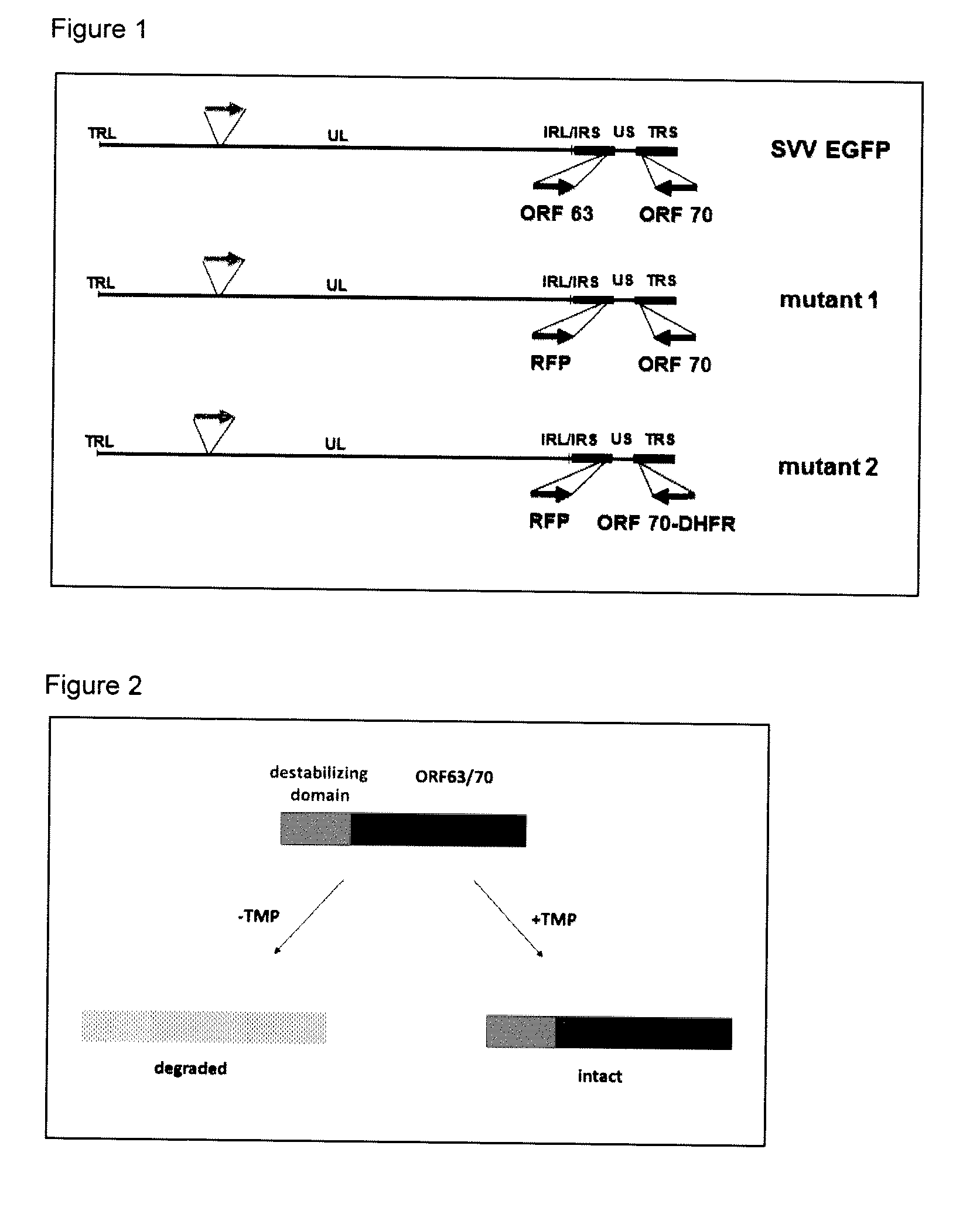
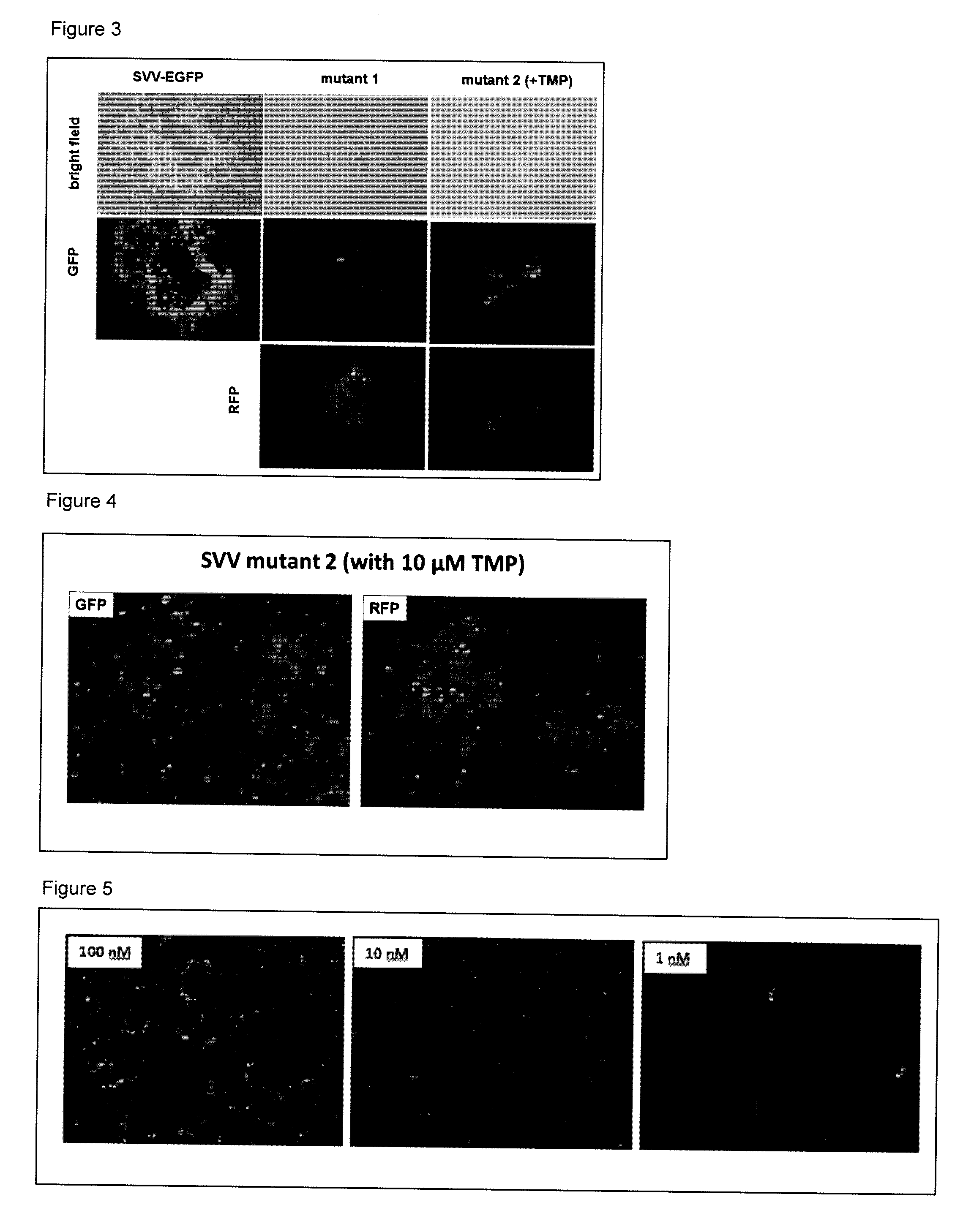
The present invention is directed to a mutated recombinant herpesvirus, e.g., varicella zoster virus (VZV) and simian varicella virus strains or HSV-1 or HSV-2 strains, vaccines containing, and methods for the construction and use thereof to elicit protective immunity in susceptible individuals, wherein the particular herpesvirus is modified to render the virus replication deficient, i.e., the virus substantially or only replicates under defined conditions, by the incorporation of at least one destabilization domain in or fused to a gene essential for herpesvirus replication. The invention particularly relates to the use of the resultant conditionally replication defective herpesviruses, e.g., a mutated VZV strains in vaccine compositions in order to immunize individuals against herpesvirus infection, e.g., in the case of VZV chickenpox and to protect against shingles and zoster, or to prevent the reactivation of VZV or other herpesvirus reactivation and the onset of shingles or another condition relating to the reactivation of another herpesvirus infection, e.g., as a consequence of advanced age, stress, inflammation, drug or other therapy, cancer, or immunodeficiency such as in HIV-AIDS or other diseases resulting in impaired T and / or B cell immunity.
Owner:UNIV OF COLORADO THE REGENTS OF
Recombinant Varicella-Zoster Virus
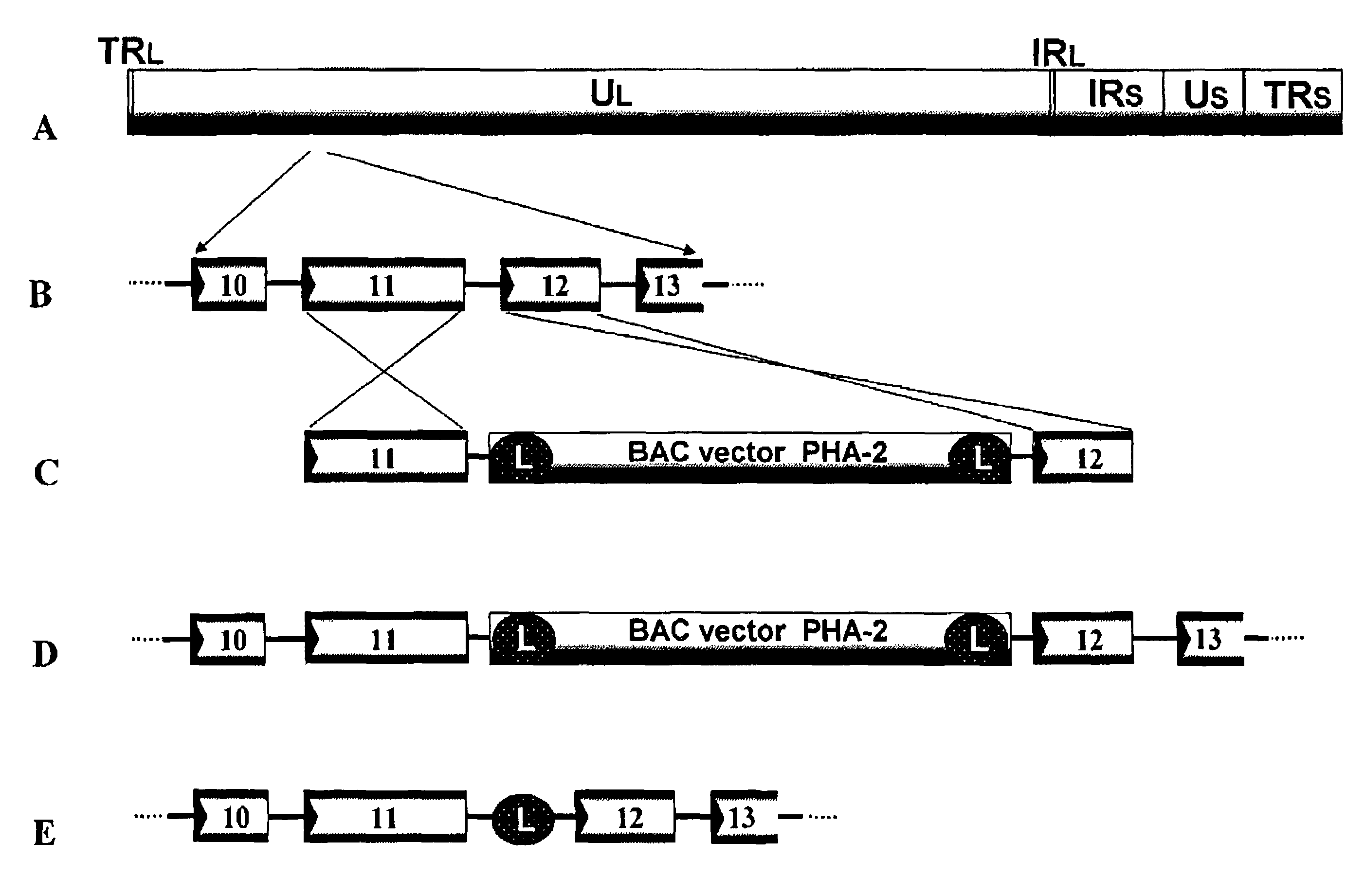
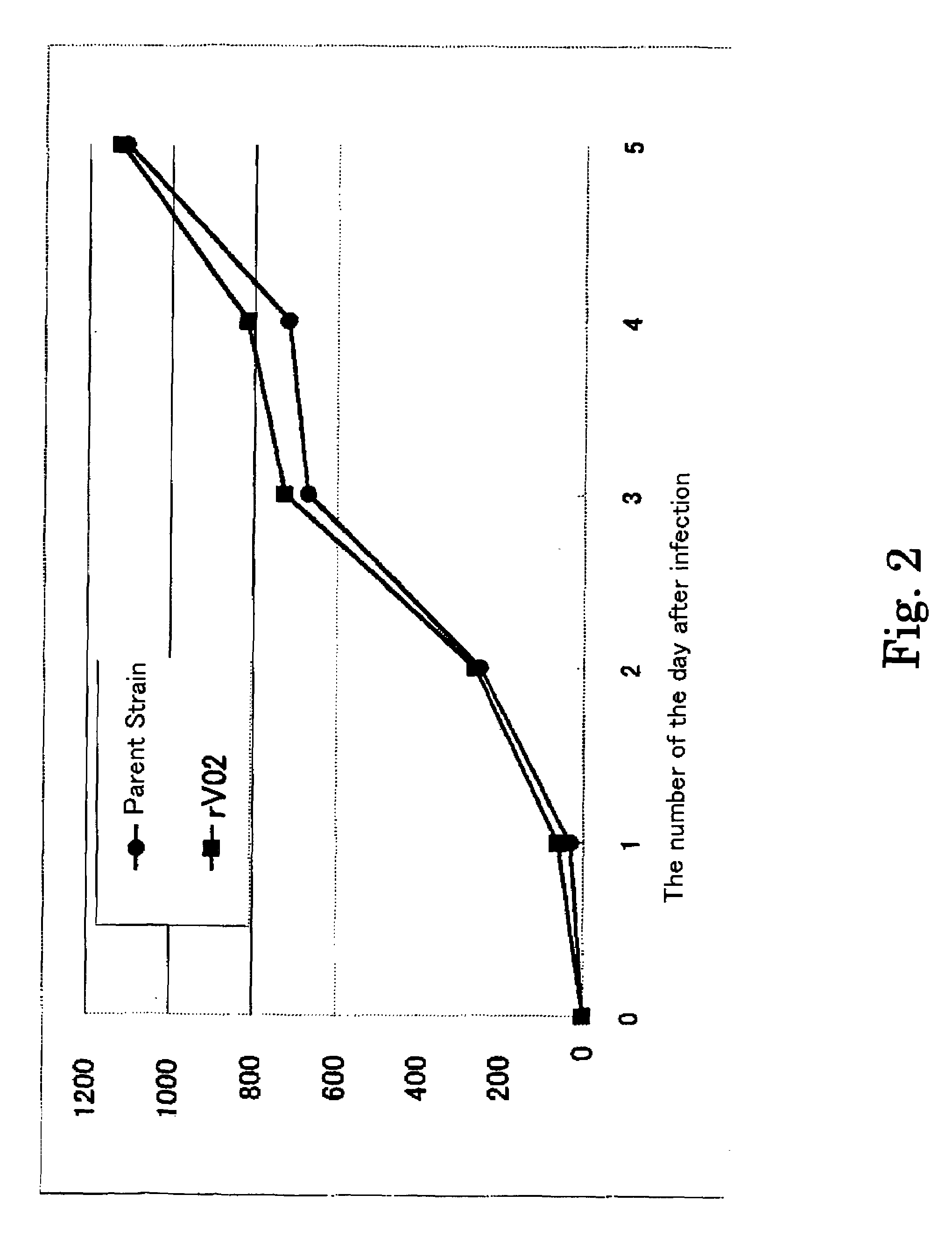
InactiveUS20110189233A1Improve accuracySecuring and ensuring effectivenessBacteriaVirus peptidesVaricella zoster virusGenome
A recombinant varicella-zoster virus; a process for producing the same; a pharmacological composition containing recombinant varicella-zoster virus; a vector containing a genomic gene of varicella-zoster virus and BAC vector sequence; cells containing the above vector; a fragment capable of homologous recombination with a genome of varicella-zoster virus; and a nucleic acid cassette containing the BAC vector sequence. For these, there is provided a process for producing recombinant varicella-zoster virus, comprising use of the BAC vector sequence.
Owner:THE RES FOUND FOR MICROBIAL DISEASES OFOSAKA UNIV
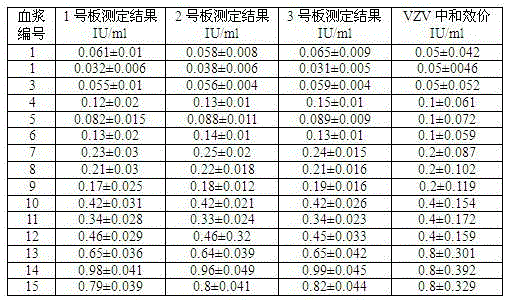
Kit capable of determining titer of neutralizing antibodies of varicella-zoster viruses and production method thereof
ActiveCN104360055AIntegrity guaranteedGuaranteed validityMaterial analysisViral glycoproteinChickenpox
The invention relates to the technical field of in-vitro diagnostic reagents and particularly relates to a kit capable of determining the titer of neutralizing antibodies of varicella-zoster viruses and a production method thereof. The kit adopts cells infected by VZV as antigens, is coated and fixed in holes of a 96-pore plate, the neutralizing antibodies in the sample to be determined are captured by using a large amount of virus glycoprotein (neutralizing glycoprotein) carried on the surfaces of the cells, simultaneously the integrity of the cells is maintained; and the other virus antigens inside are not contacted with the sample, so that the specificity of the neutralizing antibodies in determination can be guaranteed. The kit can be directly used for fast determination of the titer of the neutralizing antibodies in VZV on an automatic biochemical analyzer or an enzyme labeling instrument; the determination process is simple and fast, the flux of the determined sample is high and quantitative determination can be realized.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
Immunoreactive regions of glycoprotein gpII of varicella zoster virus (VZV)
InactiveUS6960442B2Peptide/protein ingredientsVirus peptidesVaricella-zoster virus infectionGlycoprotein
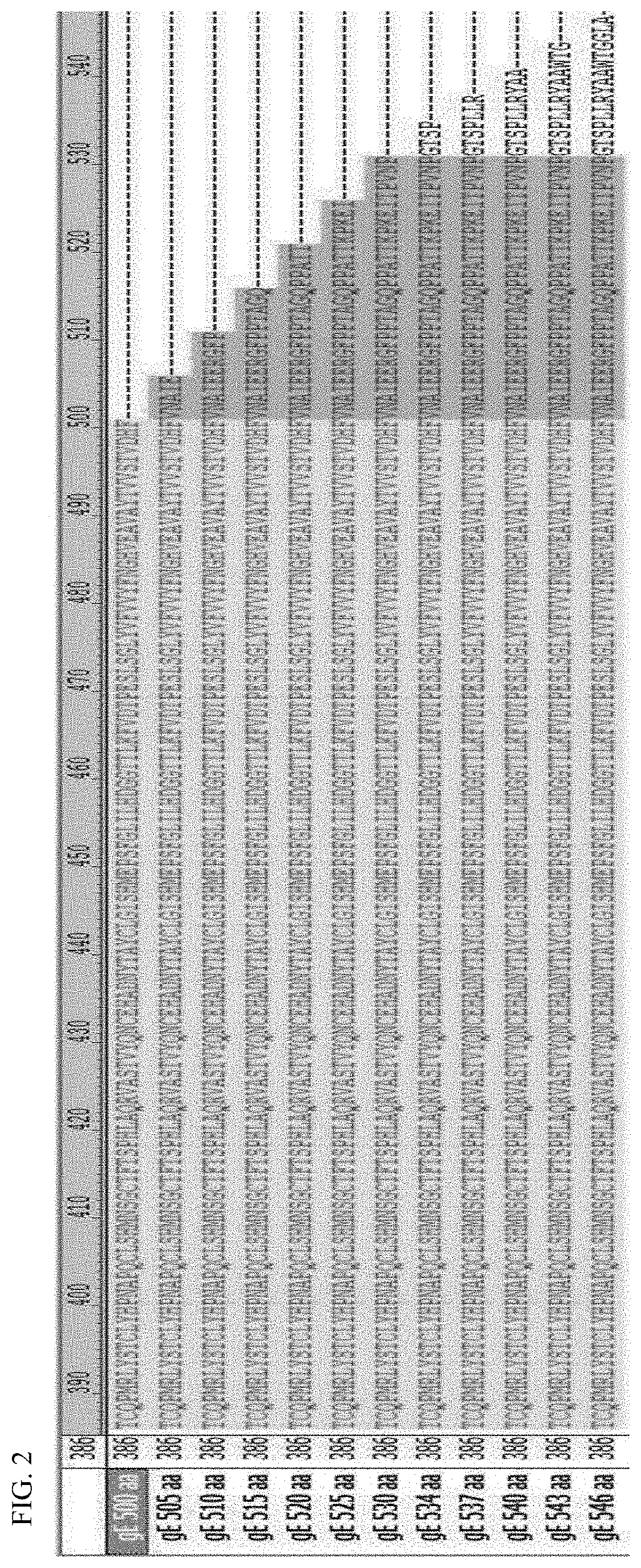
The present invention relates to immunoreactive peptides that are homologous with the region encompassing amino acid positions 450 to 655 of glycoprotein II of varicella zoster virus. In this context, preference is given to those peptides corresponding to segments of amino acids 505 to 647, 517 to 597, 535 to 584 or 545 to 582. The immunoreactive peptides are useful for methods of diagnosing varicella zoster virus infection.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS PRODS
Varicella-zoster virus gE protein mutant and expression method thereof
The invention relates to a varicella zoster virus gE protein mutant and an expression method thereof. Specifically, the invention discloses a protein with immunogenicity and a coding gene thereof. Theinvention also discloses a preparation method of the protein with immunogenicity.
Owner:IMMUNE PATH BIOTECHNOLOGY SUZHOU CO LTD
Varicella-zoster virus vaccine and application thereof
PendingCN114767844AImprove securityEnhance immune responseViral antigen ingredientsAntiviralsCholesterolHerpes zoster virus
The invention provides a varicella-zoster virus vaccine and application thereof, and belongs to the technical field of vaccines. The varicella-herpes zoster virus vaccine comprises liposome nano-particles, herpes zoster virus glycoprotein E and an adjuvant, wherein the herpes zoster virus glycoprotein E and the adjuvant are entrapped in the liposome nano-particles; the adjuvant comprises triterpenoid saponin. In the invention, after the herpes zoster virus glycoprotein E (gE) is wrapped by the liposome nanoparticles, antigen presenting cell phagocytosis and efficient antigen delivery can be effectively promoted, and sustained release of the vaccine is realized to continuously stimulate a body to generate specific cellular immune response aiming at VZV-gE. The triterpenoid saponin used in the invention can effectively realize cross presentation of antigen herpes zoster virus glycoprotein E and induce antigen-specific cellular immune response. Moreover, cholesterol rich in the lipid nanoparticles can effectively neutralize the cytotoxicity of the triterpenoid saponin, so that the safety of the vaccine is ensured.
Owner:TAIZHOU BIVO BIOTECH CO LTD
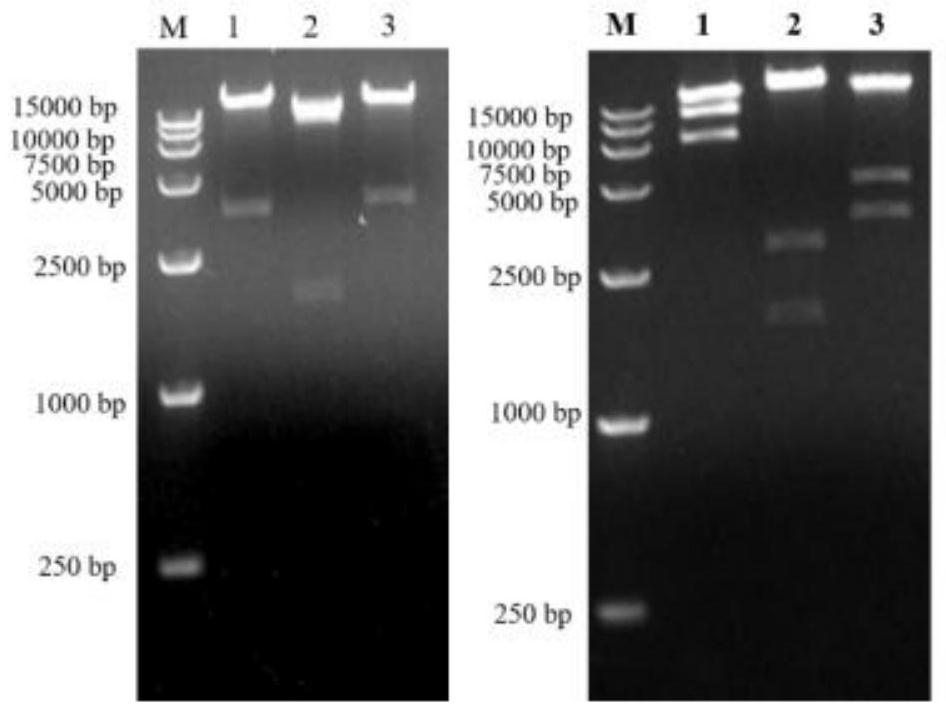
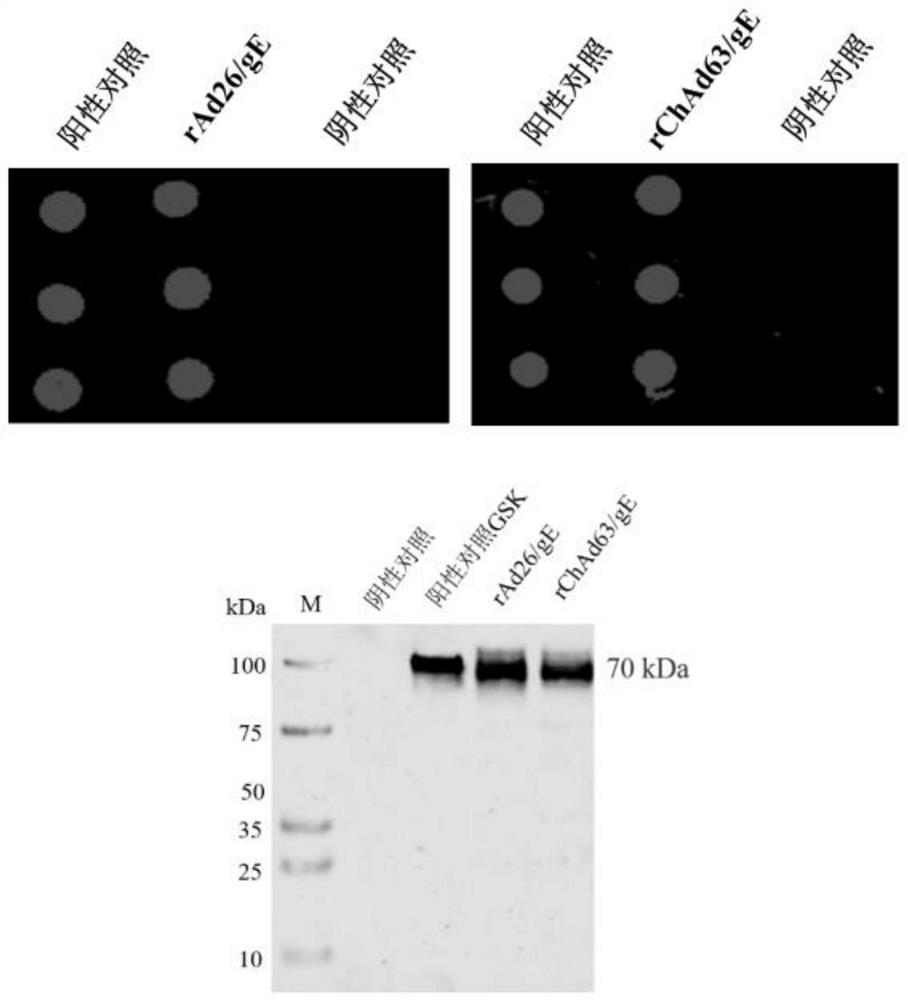
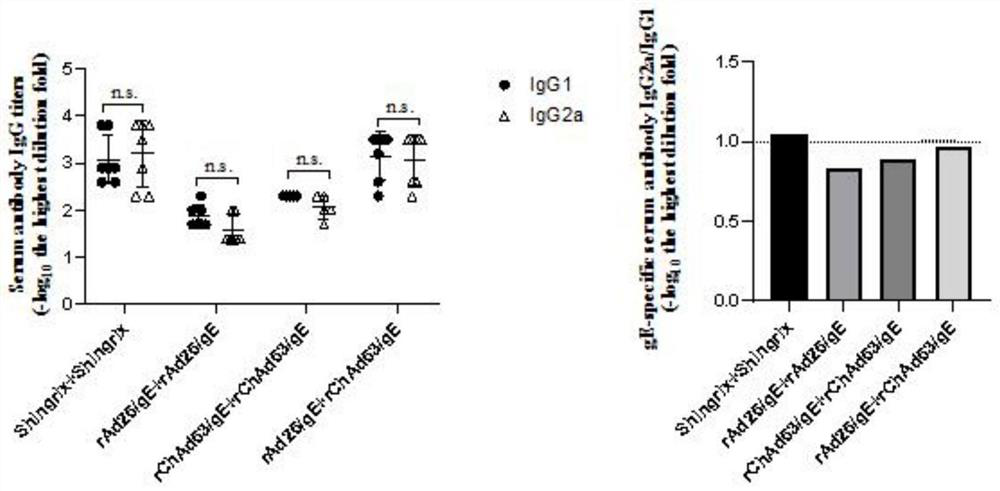
Two recombinant adenoviruses for expressing gE protein of varicella-zoster virus and application
PendingCN114163503AStrong cellsHighly reactiveViral antigen ingredientsVirus peptidesHeterologousNucleotide
The invention provides two recombinant adenoviruses for expressing gE protein of varicella-zoster virus and application, and relates to the technical field of biological medicine. The invention provides two recombinant adenoviruses prepared on the basis of the nucleotide sequence of the varicella-zoster virus gE protein, and the recombinant adenoviruses capable of expressing the varicella-zoster virus gE protein are homologous or heterologous combined as the herpes zoster virus vaccine. The immunopotentiator can induce the body to generate strong cellular and humoral immune response in a short time on a mouse.
Owner:BEIJING JIAOTONG UNIV
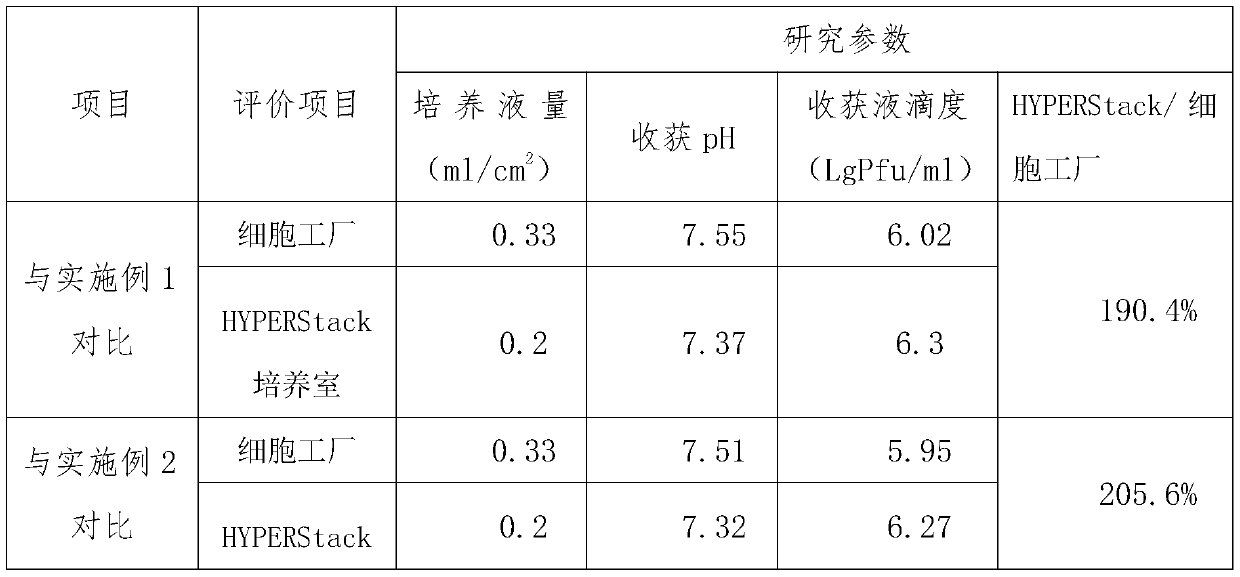
High-yield varicella virus culture method
InactiveCN111484985ASolve the exchange problemSolve the technical problem of low titer outputMicroorganism based processesDsDNA virusesChickenpoxCell growth
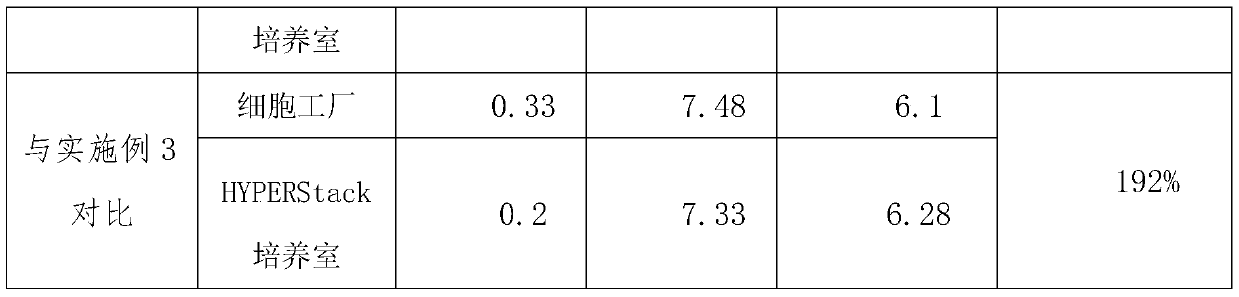
The invention discloses a high-yield varicella virus culture method. The method specifically comprises the following steps of: S1, cell resuscitation: carrying out cell resuscitation passage on a working cell bank MRC-5 from ATCC, taking an M199-801 culture solution containing 10-20% of newborn calf serum as a cell growth solution, carrying out culture at 37 DEG C with 5% of CO2, and after 3-4 days, enabling cells to grow all over a single layer and preparing for passage of the next generation; S2, cell passage: washing the surfaces of the cells twice by using a 0.01 mol / L PBS (Phosphate Buffer Solution); adding a proper amount of a pre-heated EDTA-Trypsin digestive juice; observing a gap on a cell surface by naked eyes; removing digestive juice, adding a small amount of a cell culture fluid, blowing and beating to uniformly disperse cells, carrying out passage according to a ratio of 1: 4-1: 8, finally carrying out passage on the cells into a Corning HYPERStack culture room, supplementing a 5-10% newborn calf serum M199-801 culture solution with a concentration of 0.2-0.3 ml / cm<2>, and culturing at 37 DEG C with 5% CO2. The invention relates to the technical field of biological medicines. According to the high-yield varicella virus culture method, under the production scale, after the method is adopted, the production yield is increased by about 100%, and the production cost is reduced by about 40%-50%.
Owner:JIANGSU JINDIKE BIOTECHNOLOGY CO LTD
Varicella-zoster virus mRNA vaccine as well as preparation method and application thereof
PendingCN114854772AStable structureImprove translation efficiencyVirus peptidesAntiviralsChickenpoxTGE VACCINE
The invention relates to the field of nucleic acid vaccines, in particular to a varicella-herpes zoster mRNA vaccine and a preparation method and application thereof. The mRNA for coding the varicella-zoster virus antigen provided by the invention contains a coding region for coding glycoprotein E of the varicella-zoster virus, and the mRNA can induce specific antibody response and CD4 + T cell response aiming at the glycoprotein E when being delivered into a body.
Owner:浙江君怡生物科技有限公司
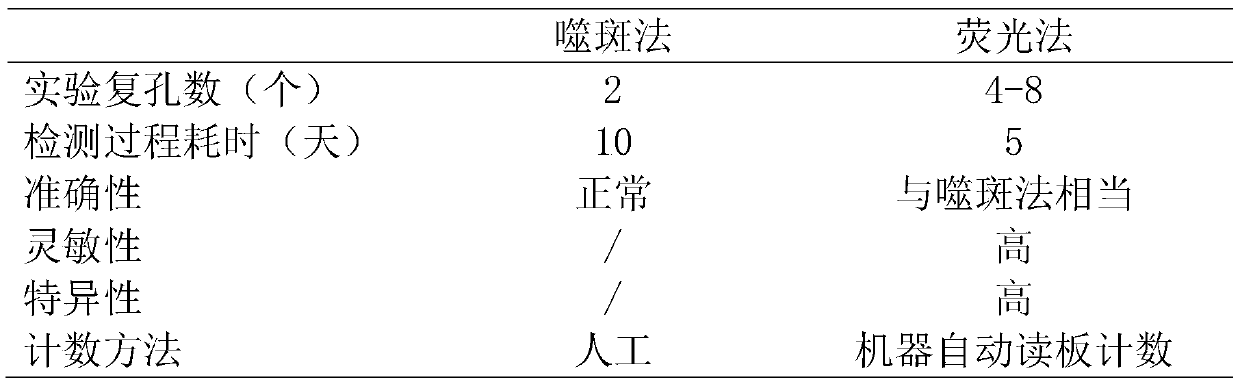
Method for rapidly detecting varicella virus titer by using fluorescence method
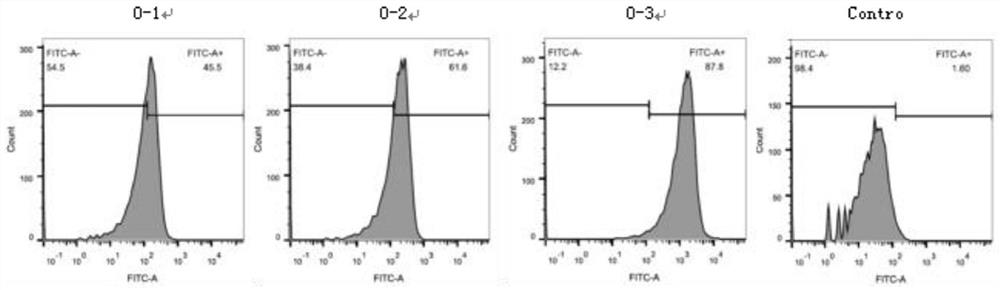
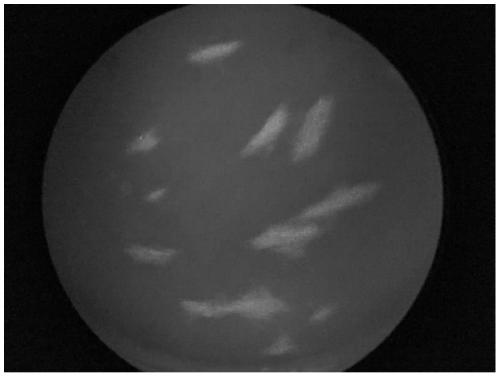
ActiveCN111579789AQuick checkAccurate detectionBiological material analysisImmunoglobulins against virusesStainingChickenpox
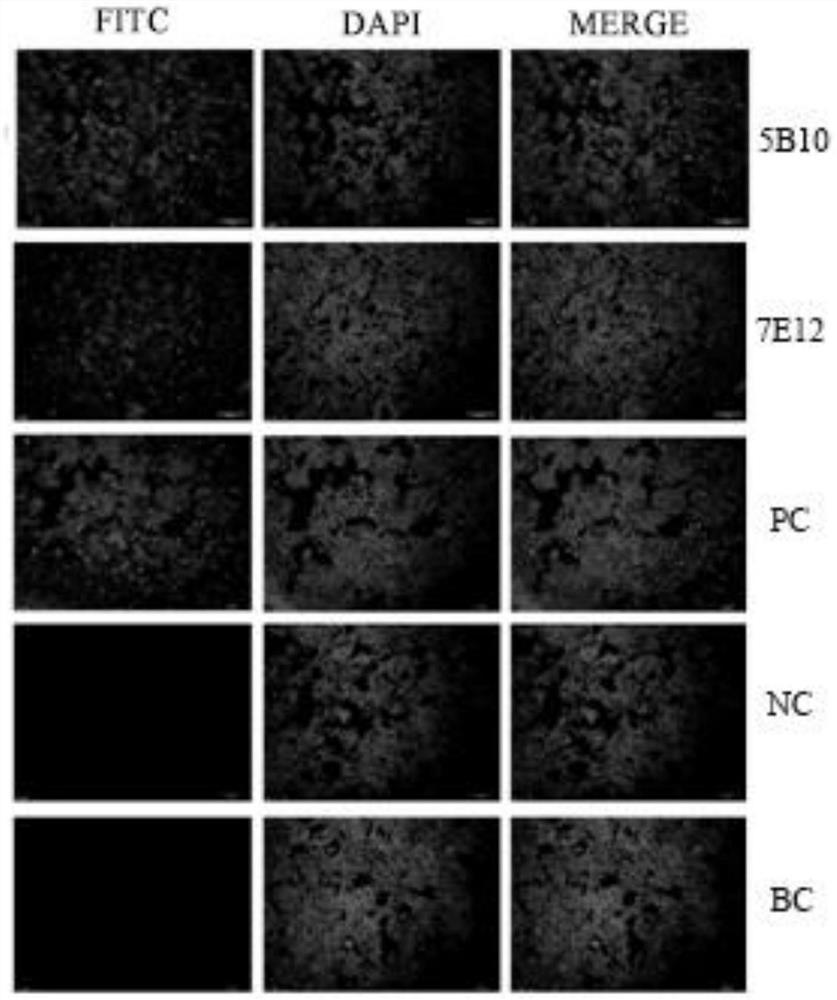
The invention provides an antibody for detecting varicella-zoster virus (VZV) titer and a related detection method. The method comprises the following steps: inoculating diploid cells into a 96-well plate, culturing, infecting a sample inoculated with unknown virus titer, culturing, adding an antibody specifically combined with VZV virus and an FITC labeled secondary antibody, carrying out fluorescence staining, and observing the infected lesion number under a microscope to obtain the virus titer of the sample. The method provided by the invention is strong in specificity, sensitive, rapid andeasy to observe, can obtain the same detection accuracy as a traditional speckle method, and can significantly shorten the detection time.
Owner:CHANGCHUN KEYGEN BIOLOGICAL PROD
Preparation method of varicella-zoster virus vaccine
InactiveCN111500548AAvoid breakingSuitable for collectionViral antigen ingredientsMicroorganism based processesChickenpoxHerpes zoster virus
The invention discloses a preparation method of a varicella-zoster virus vaccine. The preparation method comprises the following specific preparation steps: carrying out centrifugal separation on harvested cell sap, a UniFuge disposable barrel-shaped bowl body centrifugal machine is adopted for centrifugal separation, in addition, the method further comprises the procedures of cell recovery, cellpassage, virus culture, cell harvesting and centrifugal washing. According to the invention, the problems of high cell collection and washing sterile operation risk and poor bovine serum albumin washing and removal effect in the varicella-zoster virus vaccine production process in the prior art can be solved.
Owner:江苏金迪克生物技术股份有限公司
Genetically engineered cell lines and systems for propagating Varicella zoster virus and methods of use thereof
InactiveUS20060121048A1Cell receptors/surface-antigens/surface-determinantsViral antigen ingredientsHerpes zoster virusMolecular biology
The present invention provides genetically engineered cell lines, recombinant vectors, and vaccines. The present invention also provides methods for generating an in vitro system for Varicella zoster virus (VZV), and the in vitro systems generated by these methods. The present invention further provides methods for reactivating VZV, and VZV reactivated by these methods. Finally, the present invention provides a method of screening for an agent for treating VZV infection.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Composition of starwort total glycosidoprotein, preparation method and uses thereof
InactiveCN101385762AGood antiviral effectEliminate side effectsPeptide/protein ingredientsAntiviralsEnterovirusCondyloma virus
At present, the whole world is a lack of clinically high-efficient and safe broad-spectrum antiviral drugs. A composite of total riboside proteosome component that is prepared from plant chickweed or another stellaria plant and extracted by two resin adsorption methods and a hydroalcohol through a hyperfiltration method, has a molecular weight of 1,000 to 100,000 and is in the form of dark brown powder is used as a safer and more high-efficient broad-spectrum antiviral natural drug. The total protein of the total riboside proteosome component takes up 15% to 25% in the chemical construction, and is made up of 17 amino acids, such as asparaginic acid, glutamic acid, serine, histidine, cystine, methionine, isoleucine and the like in proportions; the proportion of sugar comprises ribose, glucose, galactose and arabinose, and total ribonucleotide takes up 5% to 10% and comprises uracil, thymine, thymidine, guanosine, 2-chloro-adenosine. The composite can be used as a drug for treating virus diseases, such as AIDS virus, hepatitis virus, respiratory syncytial viruses like influenza virus, parainfluenza virus, adenovirus, and the like, including highlypathogenic avian influenza virus, and condyloma virus, enterovirus, mumps virus, herpes simplex virus, herpes zoster virus and varicella-zoster virus. Moreover, the composite has no toxicity, and can be made into more than 10 types of medicine preparations, health products and the like, while causing no environmental pollution in the production process.
Owner:朱耕新
Fluorescence-labeled varicella-zoster virus immunochromatography test paper and application thereof
PendingCN114324878ASpecific recognitionQuick checkVirus peptidesImmunoglobulins against virusesAntigenChickenpox
The invention provides immunochromatography detection test paper for a fluorescently-labeled varicella-zoster virus and application of the immunochromatography detection test paper. The test paper comprises a supporting bottom plate and an adsorption layer fixed on the supporting bottom plate, wherein the adsorption layer sequentially comprises a sample pad, a combination pad, a nitrocellulose membrane and a water absorption pad from a test end; the nitrocellulose membrane contains a detection line T and a quality control line print; a monoclonal antibody 7E12 marked by quantum dots is marked on the combination pad; a monoclonal antibody 5B10 is marked by the detection line T; sPA is marked by the quality control line. Wherein the monoclonal antibody 7E12 and the monoclonal antibody 5B10 are prepared by using a gE protein as an antigen. The glycoprotein gE monoclonal antibody can specifically recognize and resist the varicella-zoster virus glycoprotein gE protein and the varicella-zoster virus, and can be used for detecting the varicella-zoster virus glycoprotein gE and the varicella-zoster virus.
Owner:ZHENGZHOU UNIV
Antigen variant of varicella zoster virus and use thereof
ActiveUS20210187099A1High expressionImproving immunogenicityViral antigen ingredientsVirus peptidesChickenpoxVaricella-zoster virus antigen
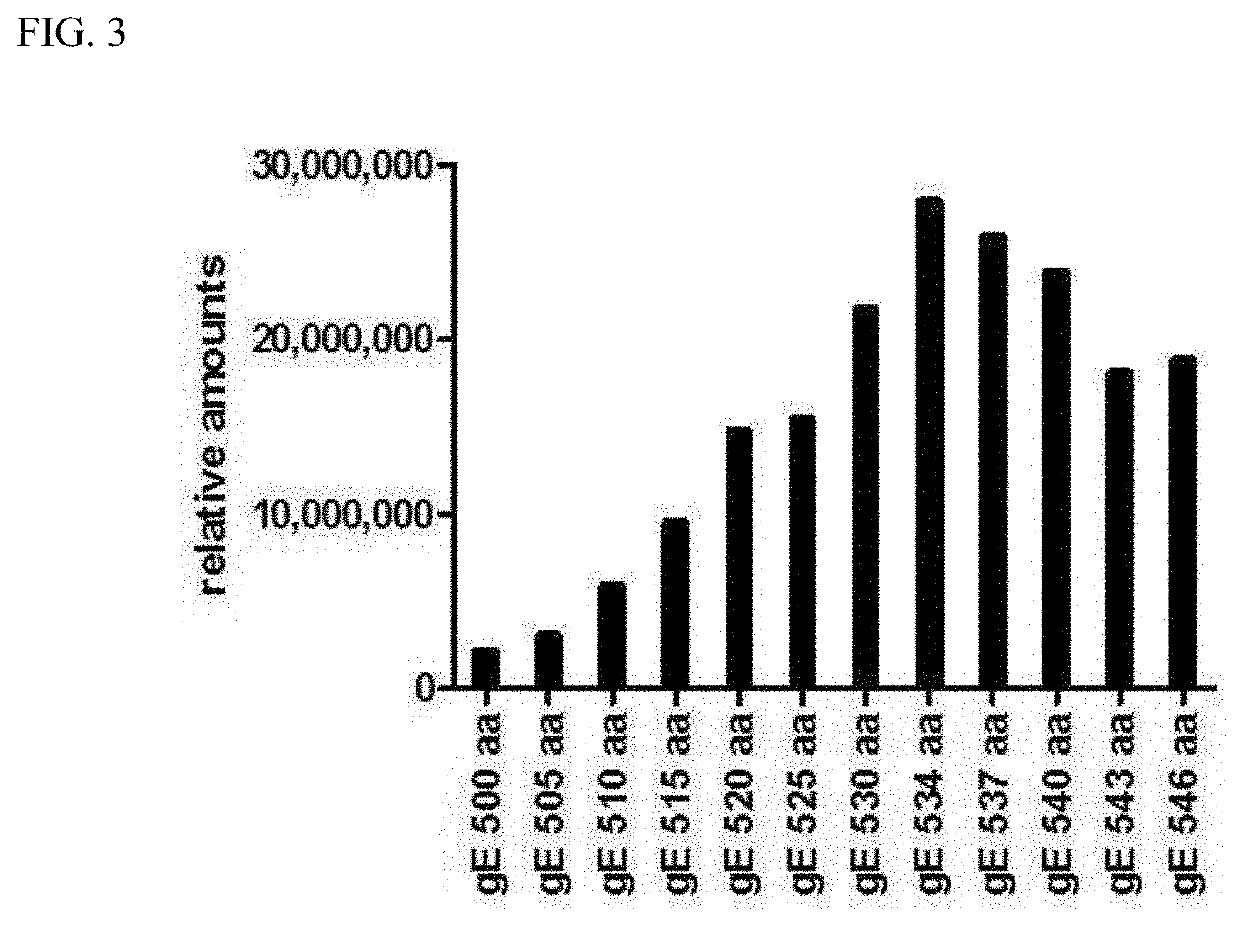
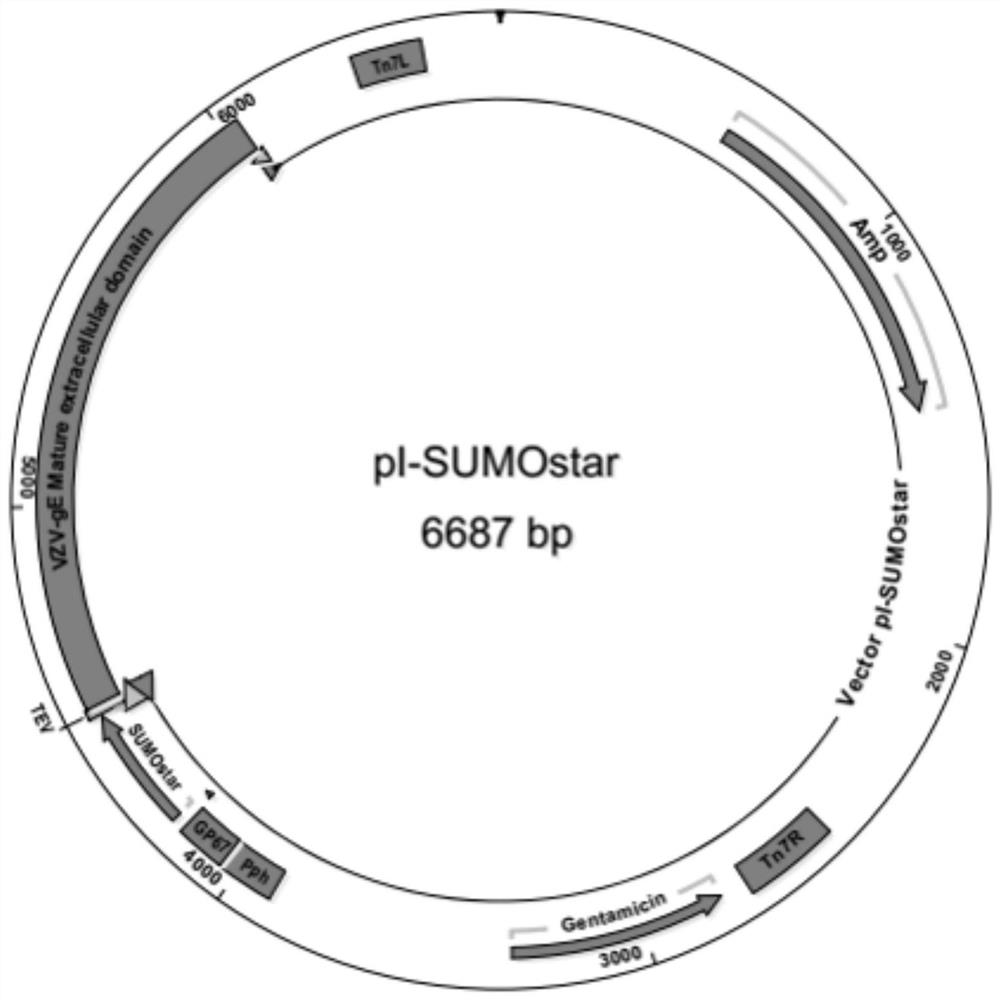
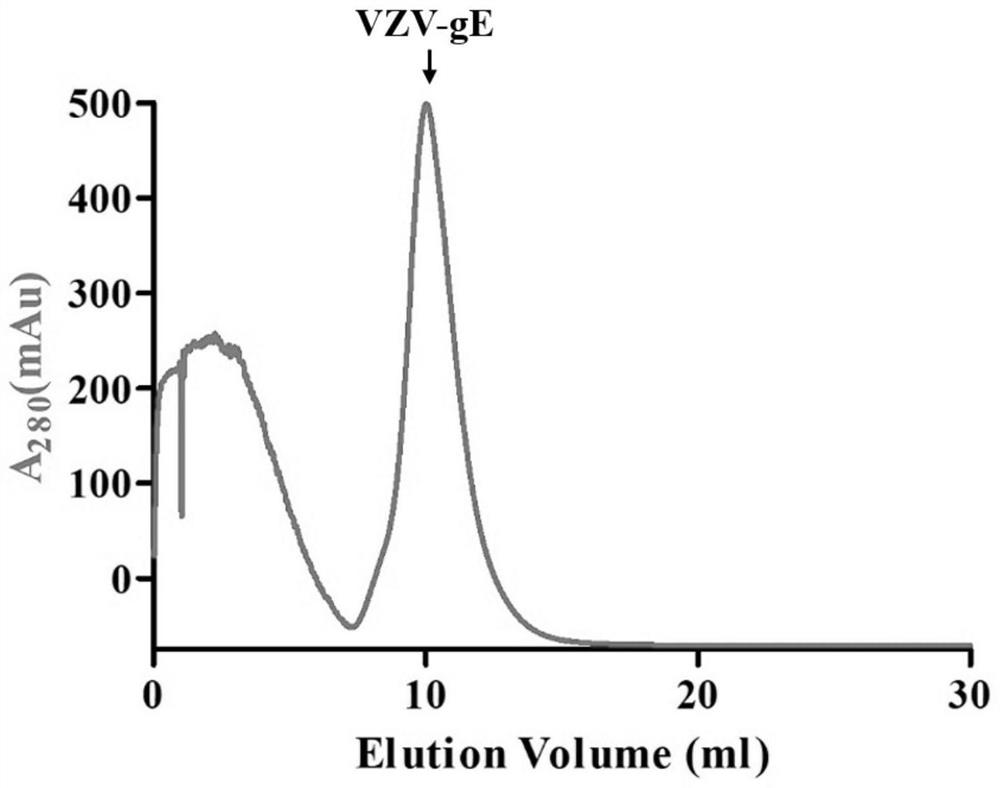
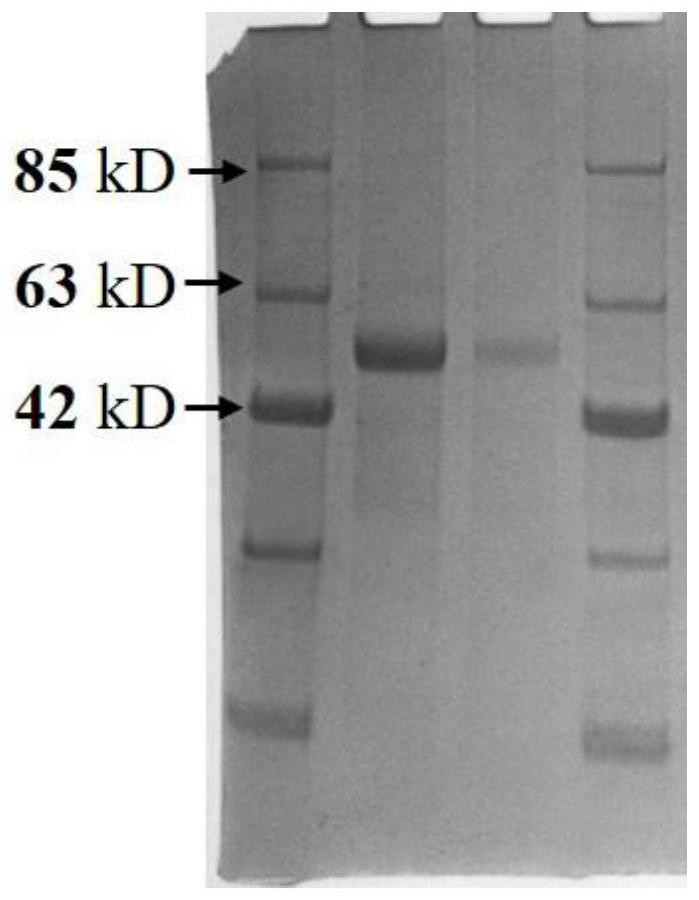
An antigen variant and a use thereof are disclosed. The antigen variant is a protein, among surface proteins (gE) of the varicella zoster virus, exhibits a high expression level and high immunogenicity, and thus, when the antigen variant is used as a vaccine composition, the vaccine composition has more excellent safety compared to a live virus vaccine, and the antigen variant exhibits a higher expression level in a host cell compared to other antigens. The antigen variant is useful as a vaccine for preventing or treating chicken pox or herpes zoster caused by the varicella zoster virus.
Owner:MOGAM INST FOR BIOMEDICAL RES
VZV infection diagnosis and detection kit based on chemiluminescence immunoassay
PendingCN114150020AReduce false negativesReduce false positivesChemiluminescene/bioluminescenceVirus peptidesDiseaseInfection diagnosis
The invention relates to a VZV infection diagnosis and detection kit based on a chemiluminescence immunoassay method. According to the present invention, with the automated chemiluminescence immunoassay technology, the specific varicella-zoster virus antibodies such as immunoglobulin isoforms G, A and M (IgG, IgA and IgM) can be detected so as to diagnose diseases related to varicella-zoster virus infection. The kit provided by the invention is also suitable for screening various subjects suspected of diseases or symptoms related to VZV infection.
Owner:UNIV OF SCI & TECH OF CHINA
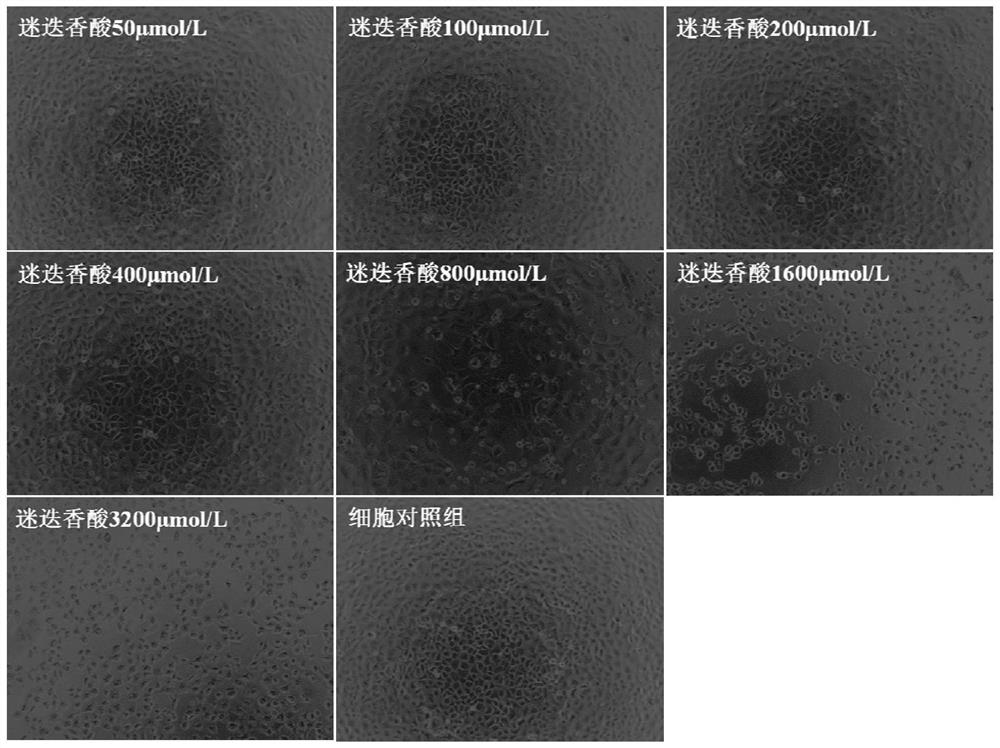
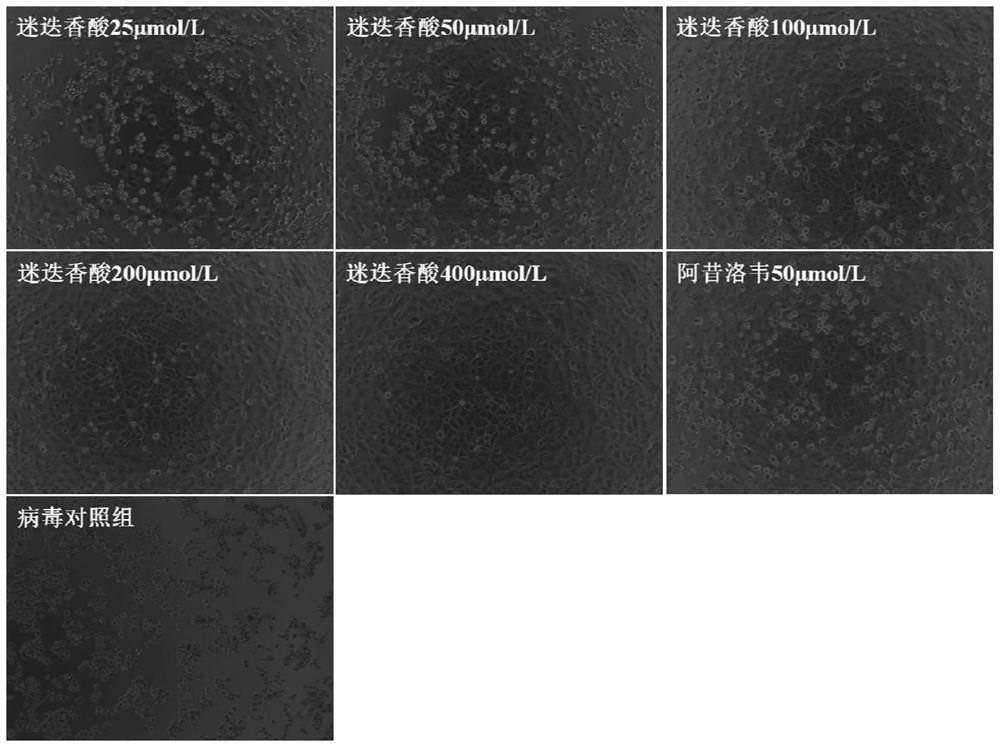
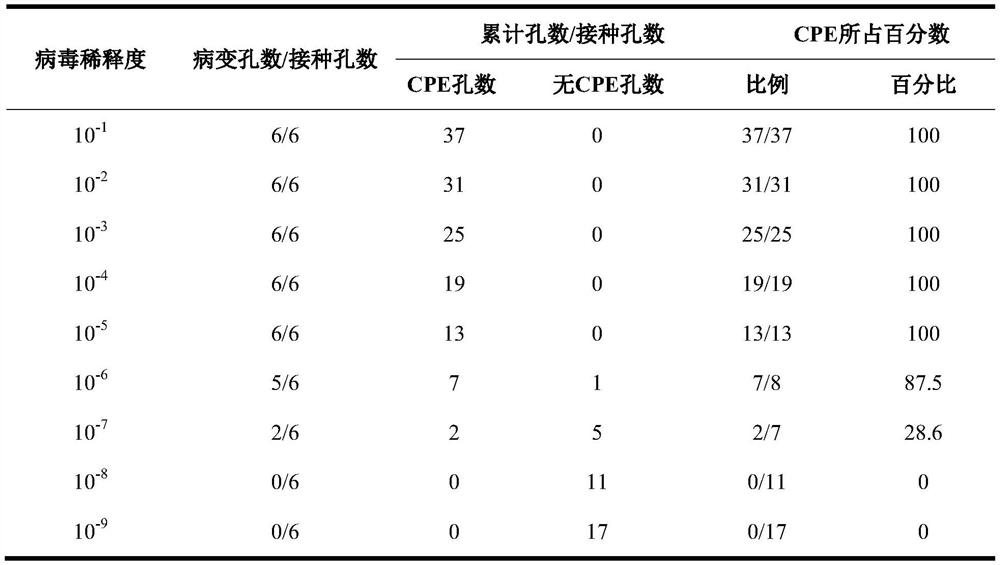
Application of rosmarinic acid in preparation of medicine for inhibiting varicella-zoster virus
The invention provides the use of rosmarinic acid in the preparation of medicines for inhibiting varicella-zoster virus. The rosmarinic acid of the present invention has significant inhibitory effect on VZV, can be used to prepare medicines for inhibiting varicella-zoster virus (VZV), and can also be used to prepare medicines for treating infectious diseases caused by varicella-zoster virus, Such diseases include chickenpox and herpes zoster, postherpetic neuralgia, varicella pneumonia, and acute cerebellar ataxia and encephalitis caused by the varicella-zoster virus, among others.
Owner:CHENGDU MEDICAL COLLEGE
Combination and application of lamp primers for detection of 4 ophthalmic infection viruses
ActiveCN106048088BMicrobiological testing/measurementMicroorganism based processesChickenpoxOPHTHALMOLOGICALS
The invention discloses an LAMP (loop-mediated isothermal amplification) primer combination for detecting four types of eye infected viruses and an application. The primer combination comprises 24 single-stranded DNA molecules shown in sequences from 1 to 24. The invention also discloses the application of the primer combination, and further provides a method for identifying herpes simplex virus type I, herpes simplex virus type II, varicella-zoster viruses or cytomegalo viruses, a method for identifying whether the to-be-detected viruses are the herpes simplex virus type I, the herpes simplex virus type II, the varicella-zoster viruses or the cytomegalo viruses, and a method for identifying whether a to-be-detected sample is infected with the herpes simplex virus type I and / or the herpes simplex virus type II and / or the varicella-zoster viruses and / or the cytomegalo viruses. With the application of LAMP primers and the method, the herpes simplex virus type I, the herpes simplex virus type II, the varicella-zoster viruses and the cytomegalo viruses can be detected rapidly and accurately.
Owner:智德科技(无锡)有限公司
A rapid detection method for varicella-zoster virus titer
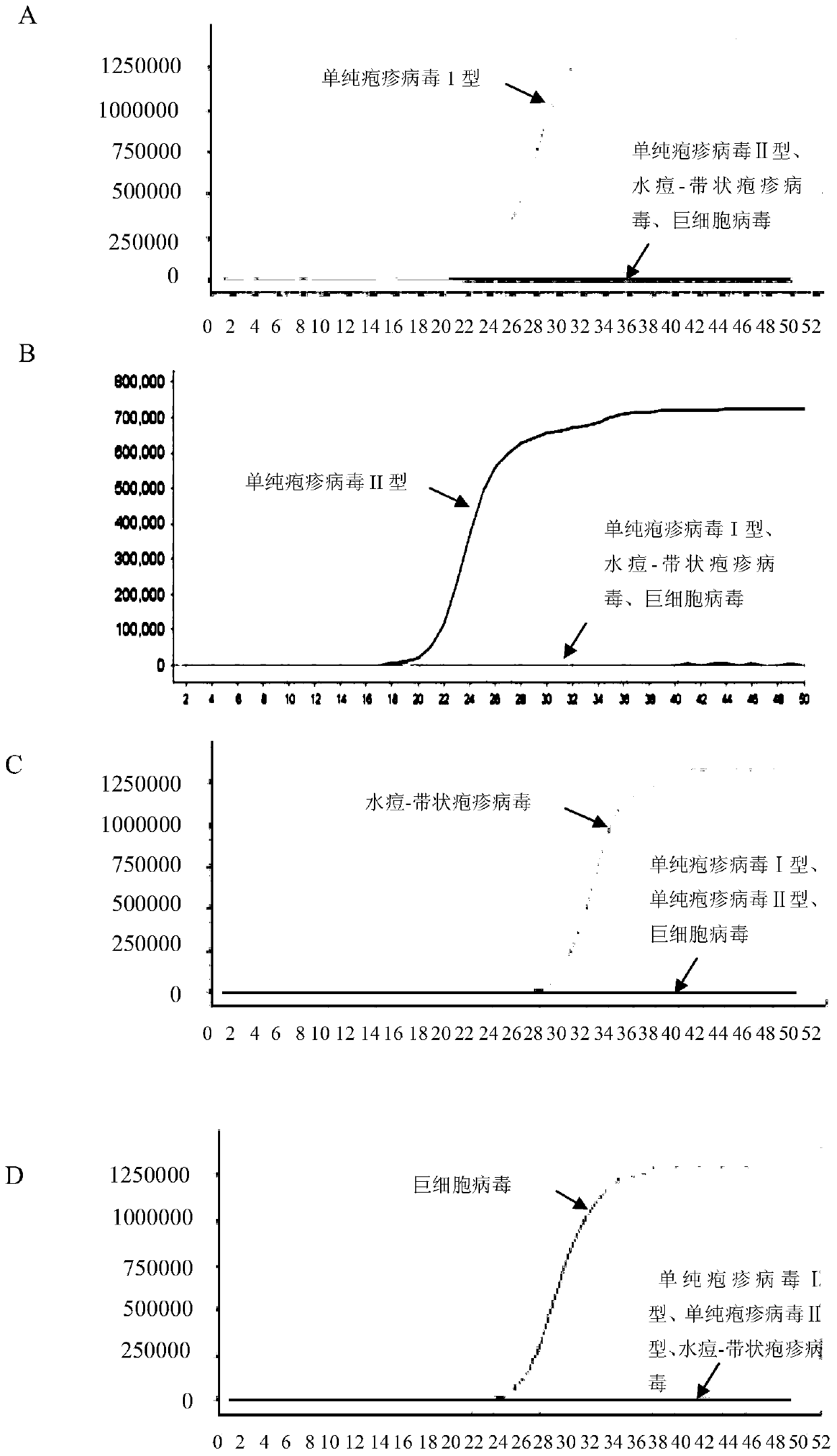
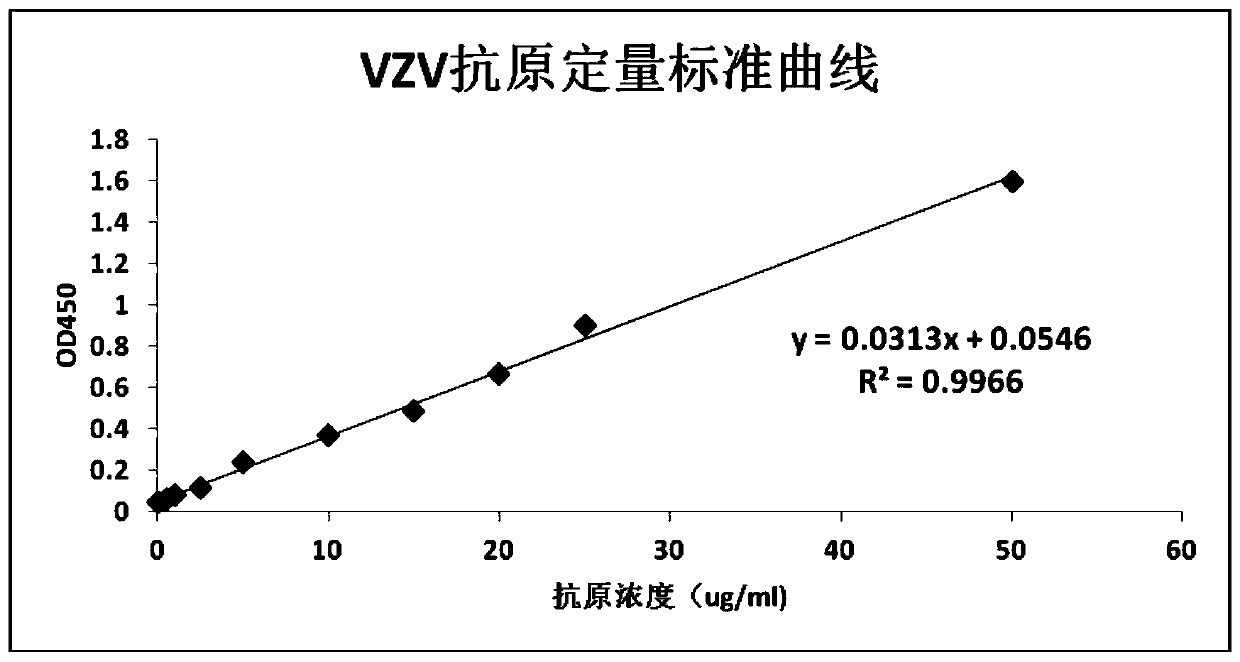
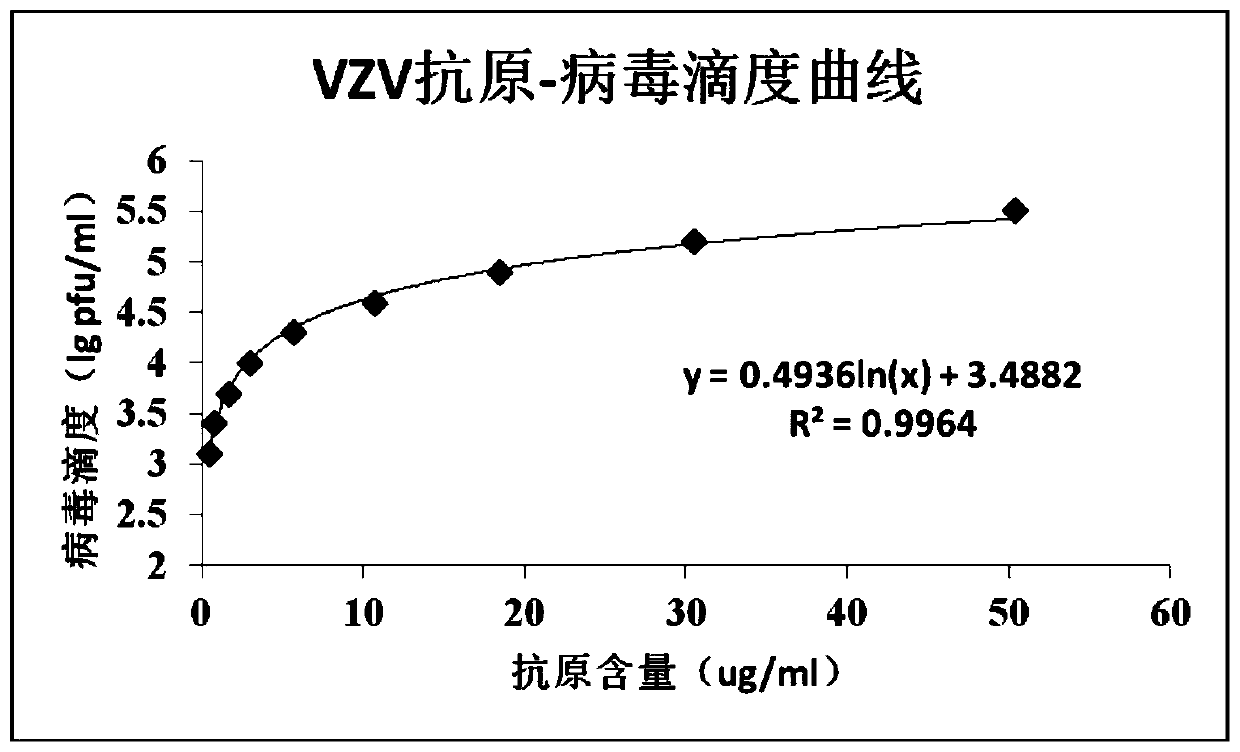
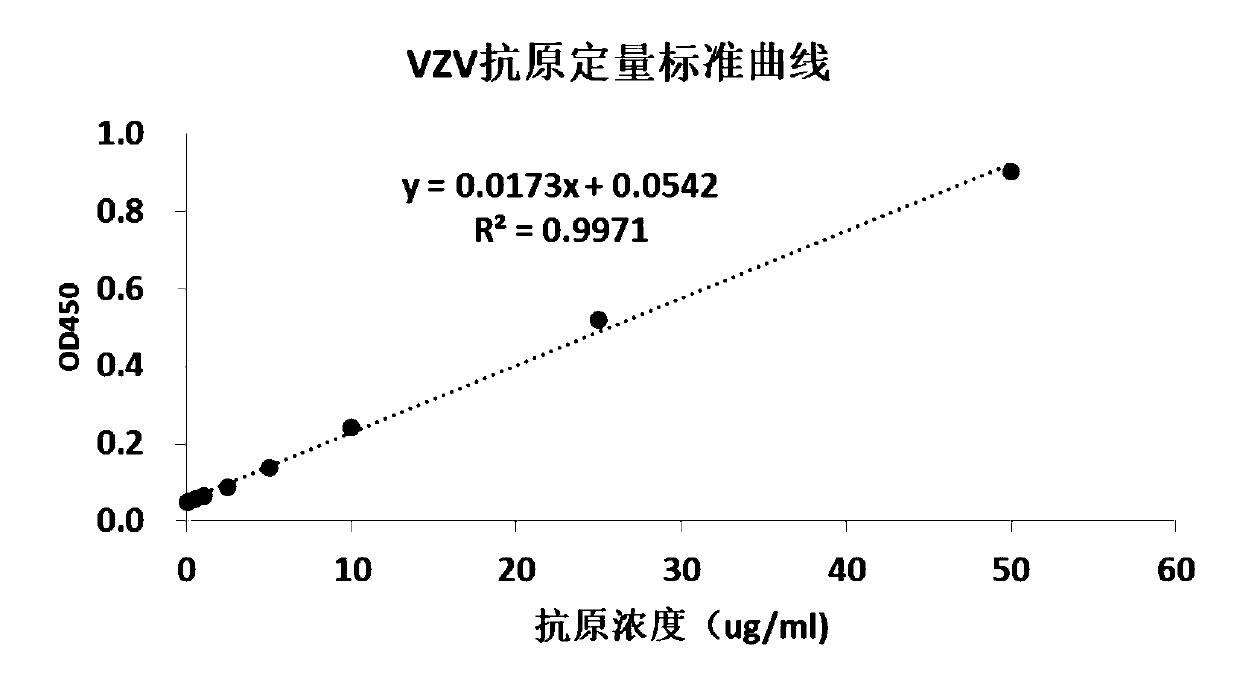
The invention aims at providing a quick detection method of virus titer of varicella zoster virus (VZV). On the basis of traditional VZV antigen double-antibody sandwich ELISA detection method, the quantitative calibration is performed by adding the VZV sample with known titer, and the virus titer of the VZV is determined by drawing a VZV antigen content titer curve. The detection method is simpleand fast in operation, compared with the traditional pfu determination method, the detection efficiency of the VZV titer is greatly improved, and the method is especially suitable for the virus quickdetermination.
Owner:CHANGCHUN KEYGEN BIOLOGICAL PROD
Application of lorarexed or pharmaceutically acceptable salt thereof in preparation of medicine for treating or preventing herpes virus infection
InactiveCN105353113BGood anti-herpes virus activityHigh activityOrganic active ingredientsAntiviralsHerpes prophylaxisChickenpox
The invention discloses application of thymidylate synthase as the target in screening anti-herpes virus drugs. thymidylate synthase can be used for screening drugs resisting Herpes simplex virus-1 (HSV-1), Herpes simplex virus-2 (HSV-2), Varicella zoster virus (VZV), EB virus (Epstein-Barr virus, EBV), Cytomegalovirus (CMV), human herpes virus type VI (HHV-6), human herpes virus type VII (HHV-7) and Kaposi's sarcoma-associated herpesvirus (KSHV) and other herpes viruses. The cell target thymidylate synthase involved in the invention can be used as a new anti-herpes virus target for drug development, and can be applied to treatment or prevention of herpes virus infection.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Anti-varicella-zoster virus siRNAs and application thereof
ActiveCN113755494AInhibition of replicationReduce loadOrganic active ingredientsAntiviralsNucleotideHerpes zoster virus
The invention discloses anti-varicella-zoster virus siRNAs. The anti-varicella-zoster virus siRNAs comprise a positive-sense strand and an antisense strand which are complementary, wherein the nucleotide sequence of the positive-sense strand is one of sequences shown in SEQ ID NO: 1, 3, 5, 7, 9, 11, 13, 15, 17, 19, 21, 23, 25, 27 and 29, or is a sequence which has the similarity of 90 percent or above with one of the nucleotide sequences of the positive-sense strand; and the nucleotide sequence of the antisense strand of the siRNAs is one of 15 sequences corresponding to the positive-sense strand respectively, or is a complementary sequence of a sequence which has the similarity of 90 percent or above with one of the nucleotide sequences of the positive-sense strand. Moreover, the invention further discloses application of the anti-varicella-zoster virus siRNAs. The siRNAs disclosed by the invention have very high biological activity, can remarkably inhibit replication of varicella-zoster virus, and have the advantages of high functionality and the like.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Duchesnea polysaccharide, as well as preparation and use thereof
InactiveCN101486771BRich sourcesBreeding is easyOrganic active ingredientsAntiviralsMonomer compositionChickenpox
The invention relates to a preparation method of polysaccharide which is extracted from plants and usage thereof, belonging to the field of biopharmaceuticals. The preparation of polysaccharide comprises extracting total polysaccharide DIP which is extracted and separated from Duchesnea indica and has clear varicella-zoster virus activity resistance property, neutral polysaccharide neutral polysaccharide DIP1 and acidic polysaccharide DIP2 in total polysaccharide, and two major active monomer compositions polysaccharide DIP30 and polysaccharide DIP 60 in acidic polysaccharide DIP2. The raw material of Duchesnea indica has wide source range, easy regeneration, easily-operated preparation method, and high reproducibility. The polysaccharide shows significant inhibition on the varicella-zoster virus, is safe, non-toxic and stable, and is a high-quality anti-viral drug candidate.
Owner:CHINA PHARM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com