Fluorescence-labeled varicella-zoster virus immunochromatography test paper and application thereof
A herpes zoster virus, immunochromatographic detection technology, applied in the direction of antiviral immunoglobulin, application, viral peptides, etc., can solve the problems of high detection equipment requirements, unsuitable basic laboratories, long detection cycle, etc., to achieve accurate virus detection. Antigen content, guiding vaccine development, and the effect of rapid diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
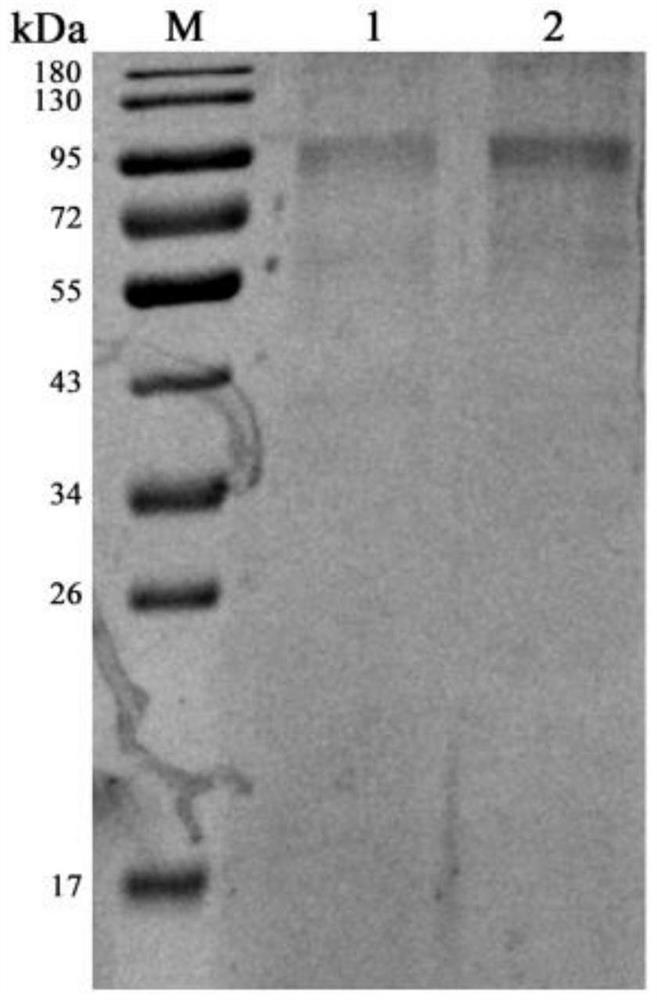
[0029] The specific embodiments of the present invention will be described in further detail below in conjunction with the examples, but the protection scope of the present invention is not limited to this; the instruments and equipment involved in the following examples are conventional instruments and equipment unless otherwise specified; the reagents involved Unless otherwise specified, they are all commercially available conventional reagents; the test methods involved are conventional methods unless otherwise specified. Example 1 Expression and purification of VZV gE protein
[0030] The VZV glycoprotein gE is the most abundant on the viral envelope, is the main antigen of the virus, and is also the main candidate antigen for the preparation of virus subunit vaccines and detection reagents. The expression of VZV gE protein and the preparation of monoclonal antibody are of great significance to the development of subunit vaccine and detection reagents for herpes zoster. M...
Embodiment 2
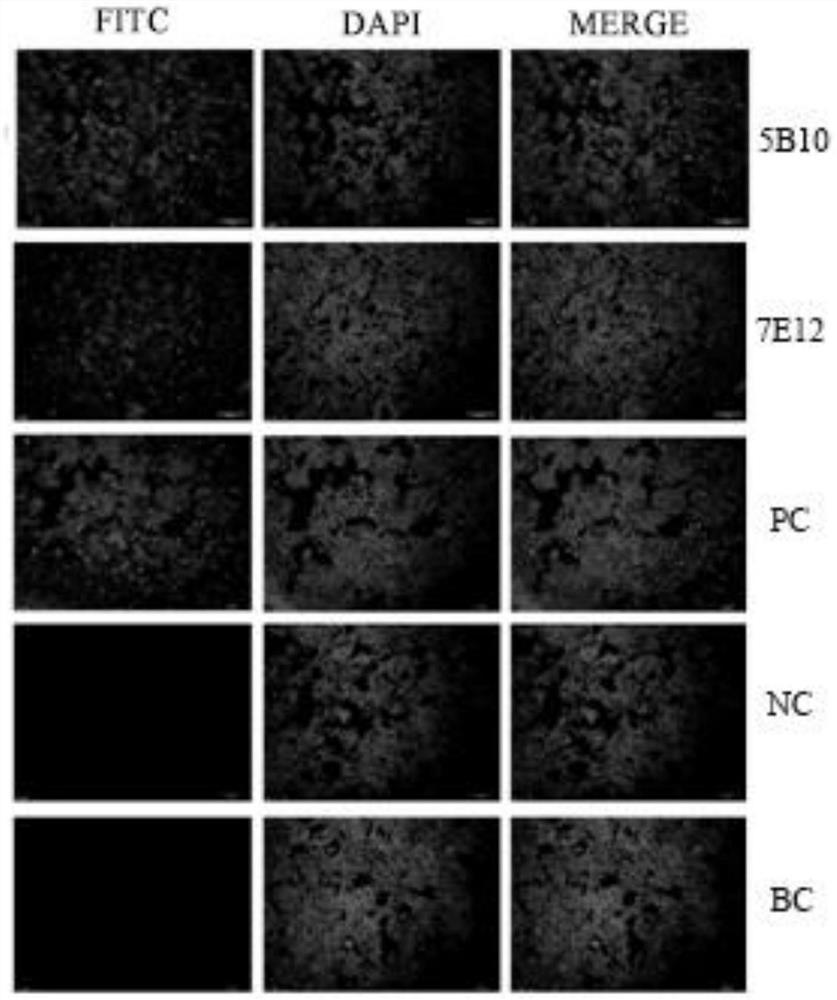
[0043] Example 2 Preparation of monoclonal antibodies
[0044] 1. Animal immunity
[0045] (1) adding Freund's complete adjuvant to the immunogen gE protein (gE protein purified in Example 1), and emulsified for the first immunization;
[0046] (2) 2 female BALB / c mice aged 4-8 weeks were immunized by subcutaneous injection at multiple points on the back, and the immunization dose was 10 μg / mice;
[0047] (3) BALB / c mice were boosted with the same method and dose after emulsification with incomplete Freund's adjuvant and immunizing antigen (gE protein purified in Example 1) every 2 weeks, and immunized 4 times in total;
[0048] (4) After 4 immunization, the tail vein blood was collected to measure the specific antibody titer against gE protein, and the mice with higher titer were selected, and 3 to 4 days before cell fusion, the method of tail vein injection was carried out, with no adjuvant. BALB / c mice were super-immunized with a dose of immunogen, and the immunization do...
Embodiment 3
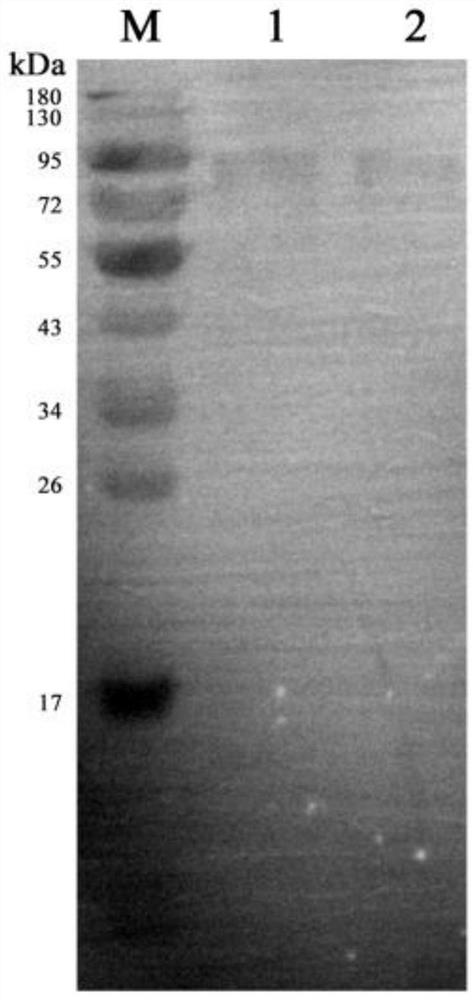
[0067] Example 3 Purification and identification of antibodies
[0068] 1. Purify antibody by saturated ammonium sulfate precipitation method. The operation method is as follows:
[0069] (1) Take 5 mL of monoclonal antibody ascites (obtained in Example 2), add 5 mL of PBS buffer, and then add 2.5 mL of saturated ammonium sulfate solution dropwise to make a final concentration of 20% (wt%) ammonium sulfate solution, while adding While stirring, after fully mixing, let stand for 30min.
[0070] (2) 8000r / min, centrifuge for 20min, discard the precipitate to remove fibrin.
[0071] (3) Add 12.5 mL of saturated ammonium sulfate solution to the supernatant, mix well, and let stand for 30 minutes.
[0072] (4) Centrifuge at 8000 r / min for 20 min, and discard the supernatant.
[0073] (5) Add 10 mL of PBS buffer to the precipitate to dissolve it, and then add 5 mL of saturated ammonium sulfate solution to make it into a 33% (wt%) ammonium sulfate solution, mix well, and let stand...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com