A rapid detection method for varicella-zoster virus titer
A technology of herpes zoster virus and virus titer, which is applied in the biological field, can solve the problems of affecting test results, long test cycle, and large impact of virus titer, and achieve the effect of shortening the time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: VZV double antibody sandwich ELISA detection kit
[0029] The VZV double antibody sandwich ELISA detection kit can be selected from the existing VZV double antibody sandwich ELISA detection kit, more preferably, the kit includes: ELISA detection plate coated with anti-VZV monoclonal antibody mAb-11, HRP enzyme labeling Anti-VZV monoclonal antibody 12-HRP, VZV antigen standard, known virus titer VZV vaccine standard, TMB chromogenic solution.
[0030] The present inventor screened hybridomas secreting VZV virus-specific monoclonal antibodies, obtained anti-VZV monoclonal antibodies mAb-11 and 12-HRP with excellent affinity and specificity, and determined the above-mentioned antibodies by competitive binding method. The affinity constant of the antibody, the results show that the affinity constant of mAb-11 is as high as 10 14 L / mol, the affinity constant of 12-HRP also reached 10 12 L / mol, both of them have very strong specific binding ability for VZV antige...
Embodiment 2
[0034] Embodiment 2: the mensuration of VZV vaccine virus titer
[0035] The present embodiment utilizes the test kit prepared in Example 1 to measure the VZV vaccine virus titer, and the specific assay method includes the following steps:
[0036] (1) Sample dilution:
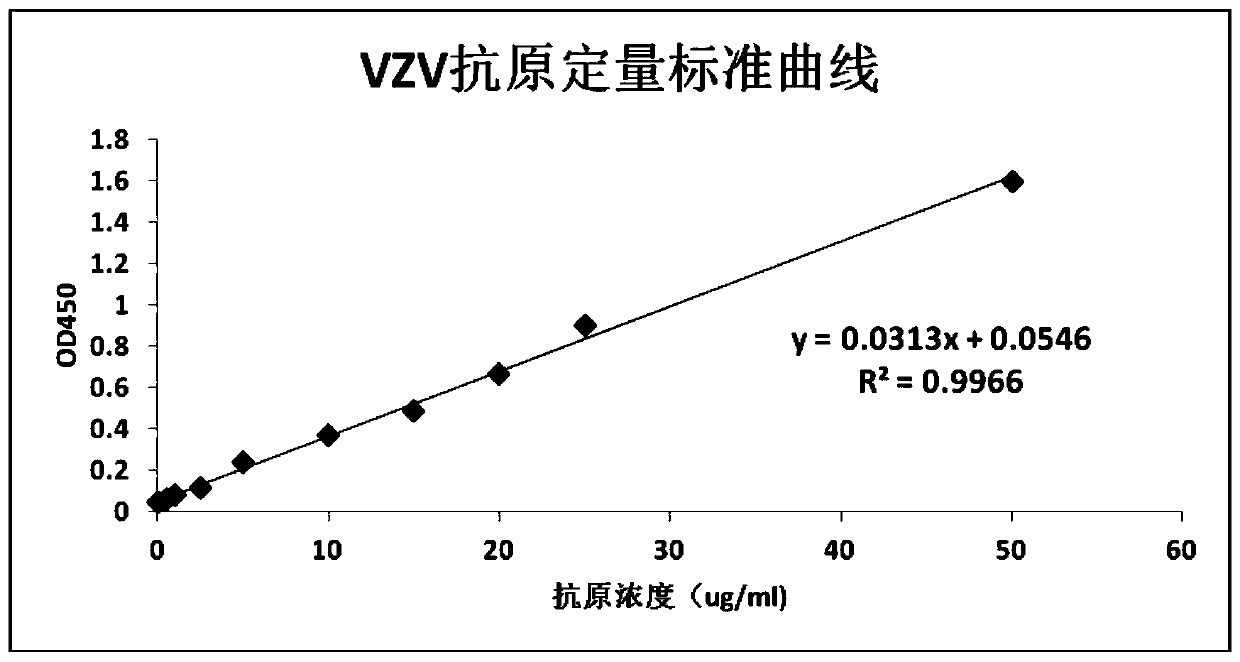
[0037] VZV standard antigen dilution: Dilute the VZV standard antigen to 50ug / ml, 25ug / ml, 20ug / ml, 15ug / ml, 10ug / ml, 5ug / ml, 2.5ug / ml, 1ug / ml, 0.5ug with PBS solution / ml, 0ug / ml;
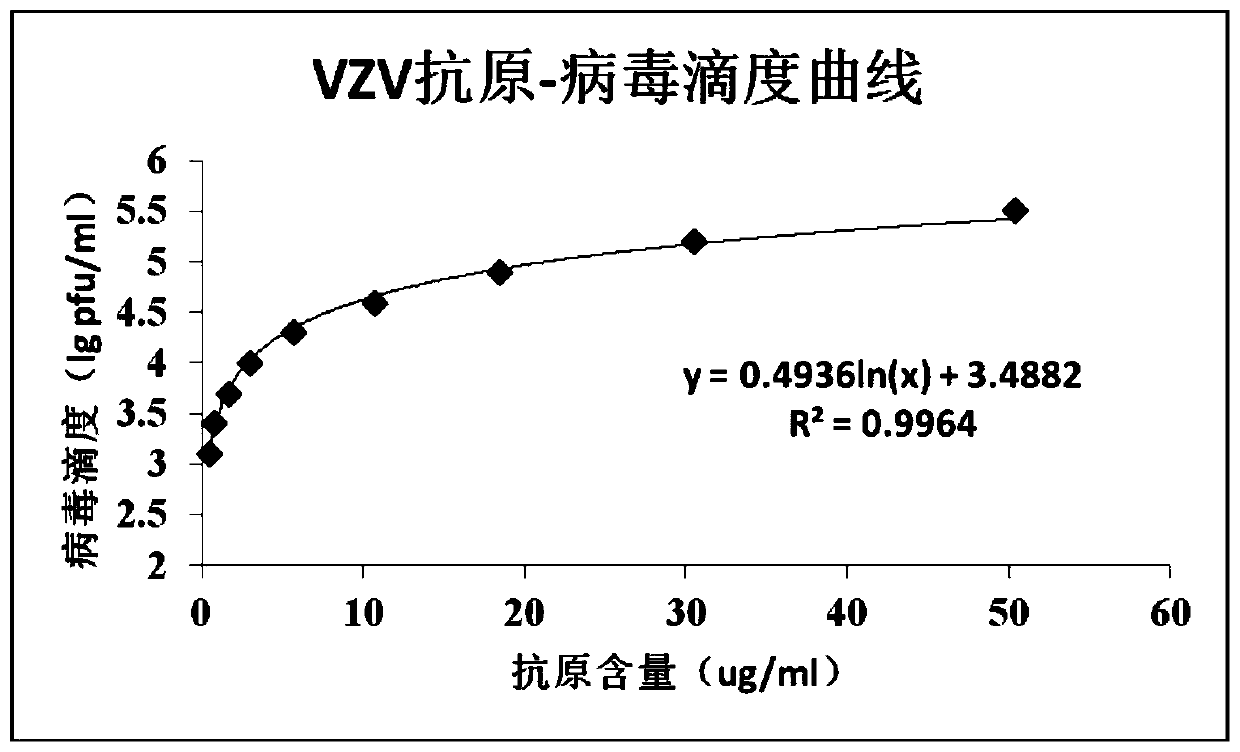
[0038] Dilution of vaccine standard with known titer: use PBS solution to make 2-fold serial dilutions of vaccine standard with known titer (5.5lg pfu / ml), every time the sample is diluted, the virus titer will be reduced on the original basis 0.3lg pfu / ml, diluted to a virus titer of 3.1lg pfu / ml.
[0039] (2) Sample addition:
[0040]Take the ELISA detection plate coated with the anti-VZV monoclonal antibody mAb-11 in the kit of Example 1 and equilibrate at room temperature; add the above-mentioned diluted sample and the sam...
Embodiment 3
[0066] Embodiment 3: the mensuration of VZV vaccine virus titer
[0067] In this embodiment, the kit prepared in Example 1 is used to measure the virus titers of four samples. The numbers of the four samples are respectively: 627-1, 627-2, 627-3, and 627-4. The specific measurement method includes the following steps :
[0068] (1) Sample dilution:
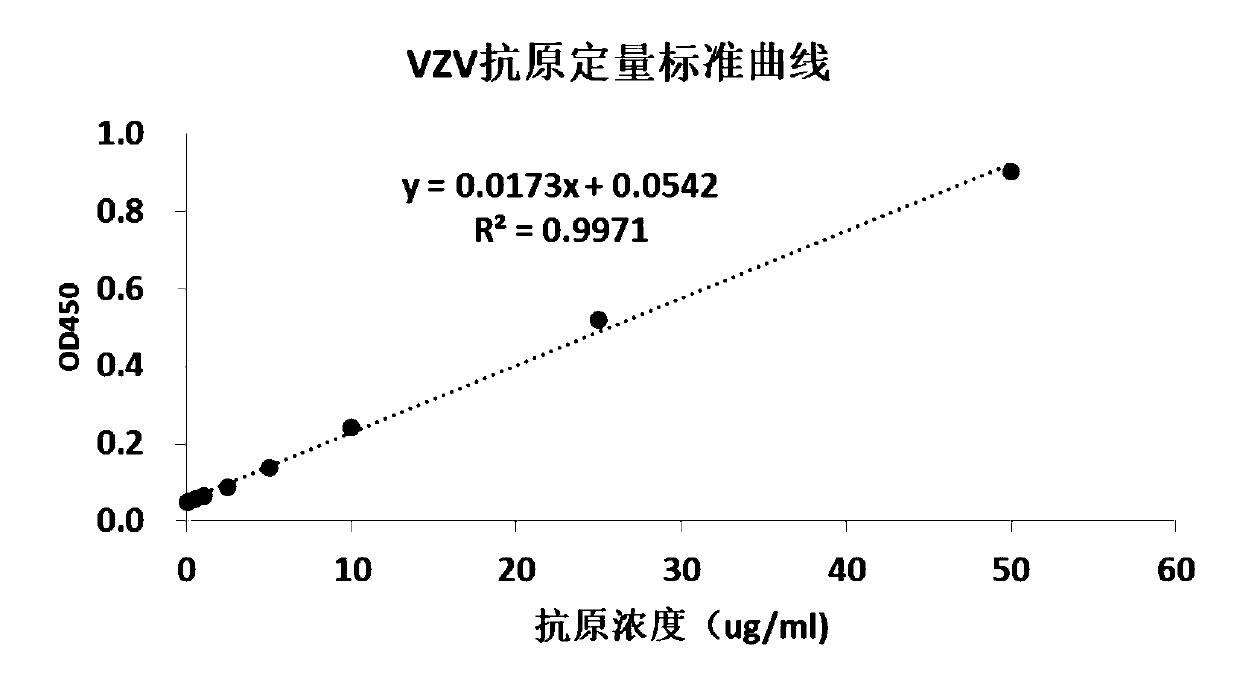
[0069] VZV standard antigen dilution: Dilute the VZV standard antigen to 50ug / ml, 25ug / ml, 10ug / ml, 5ug / ml, 2.5ug / ml, 1ug / ml, 0.5ug / ml, 0ug / ml with PBS solution;
[0070] Dilution of vaccine standard with known titer: use PBS solution to make 2-fold serial dilutions of vaccine standard with known titer (5.43lg pfu / ml), every time the sample is diluted, the virus titer will be reduced on the original basis 0.3lg pfu / ml, the virus was diluted to 3.03lg pfu / ml.
[0071] Subsequent (2) adding samples, (3) incubation, (4) washing the plate, (5) adding enzyme-labeled antibody (concentration is 0.05ug / ml), (6) washing the plate, (7) c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com