Varicella-zoster virus gE protein mutant and expression method thereof
A herpes zoster virus and mutant technology, which is applied to viruses/bacteriophages, viruses, viral peptides, etc., can solve problems such as unsatisfactory immune effect, low protein activity, and low VZVgE protein expression efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Cloning construction, expression and purification of varicella-zoster virus gE protein
[0034] 1. Selection of gE protein and synthesis (of gE gene)
[0035] Through NCBI database and literature search, a conservative truncated gE protein amino acid sequence was selected as the basis for gene optimization. In order to improve expression efficiency, only the signal peptide region and mature antigen were selected. At the same time, the inventors of the present application surprisingly found that when selecting a specific amino acid sequence as the basis for gene optimization in the process of preparing recombinant gE protein, if the 141st position of the mature antigen region is artificially modified (mutated from leucine to methionine acid), the genes and vectors designed based on the modified amino acid sequence will be able to achieve higher antigen expression. The full length of the mutated protein sequence is 546 amino acids (SEQ ID NO: 1), and the detail...
Embodiment 2
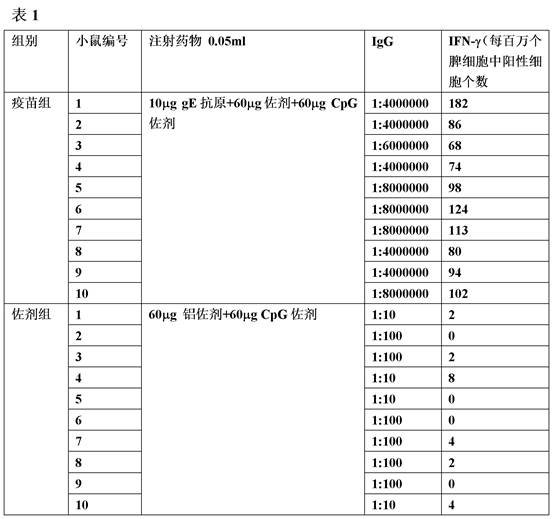
[0058] Example 2 Immunogenicity Evaluation of Recombinant Herpes Zoster Vaccine
[0059] Obtain the gE protein described in Example 1, and after conventional processing methods, such as hydrophobic chromatography, anion exchange chromatography, hydroxyapatite chromatography, ultrafiltration and nanofiltration, the protein with a purity of more than 95% can be obtained used as an antigenic protein.
[0060] In order to study the immunogenicity of the antigen prepared by the gene and carrier provided by the present invention, the above-mentioned antigen and adjuvant were sucked and formulated into a recombinant herpes zoster vaccine composition, and the C57BL / 6 mouse was used as an animal model to carry out immunization Original research. The specific method is as follows: the gE protein is used as an antigen, and aluminum phosphate and CpG ODN are used as adjuvants to inhale and prepare the recombinant herpes zoster vaccine composition. Select 6-8 week-old C57BL / 6 mice into r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com