Two recombinant adenoviruses for expressing gE protein of varicella-zoster virus and application
A technology of herpes zoster virus and recombinant adenovirus, applied in the field of biomedicine, can solve problems such as high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Construction and Identification of Recombinant Adenovirus Plasmid
[0047] 1. Construction of the shuttle plasmid
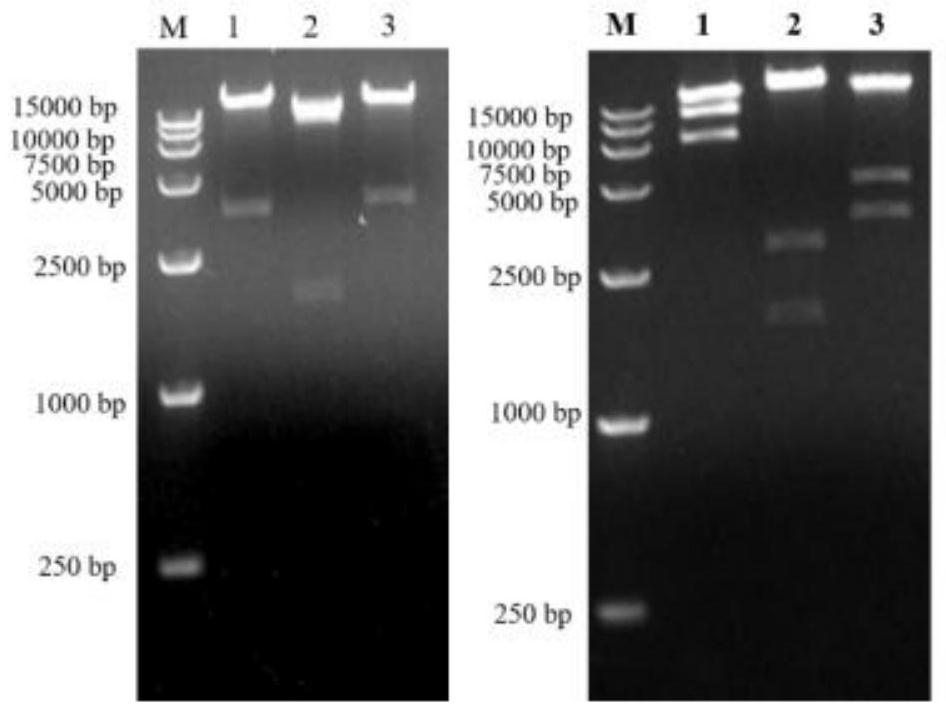
[0048] Use restriction endonucleases SalI and KpnI to double-enzyme pShuttle26 and the artificially synthesized gE nucleotide sequence respectively, recover the carrier fragment and the target fragment by gel electrophoresis, connect and transform the competent bacteria DH10B, and obtain pShuttle26 / gE.
[0049] Restriction endonucleases SalI and KpnI were used to double-digest pShuttle63 and the artificially synthesized gE nucleotide sequence, respectively, and the carrier fragment and the target fragment were recovered by gel electrophoresis, connected and transformed into competent bacteria DH10B to obtain pShuttle63 / gE.
[0050] 2. Construction of recombinant adenovirus plasmid
[0051] Digest pShuttle26 / gE with restriction endonuclease BamHI and MluI, recover the target fragment by gel electrophoresis, and single-digest pAd26 with restriction endonucl...
Embodiment 2
[0054] Rescue and expression identification of recombinant adenovirus
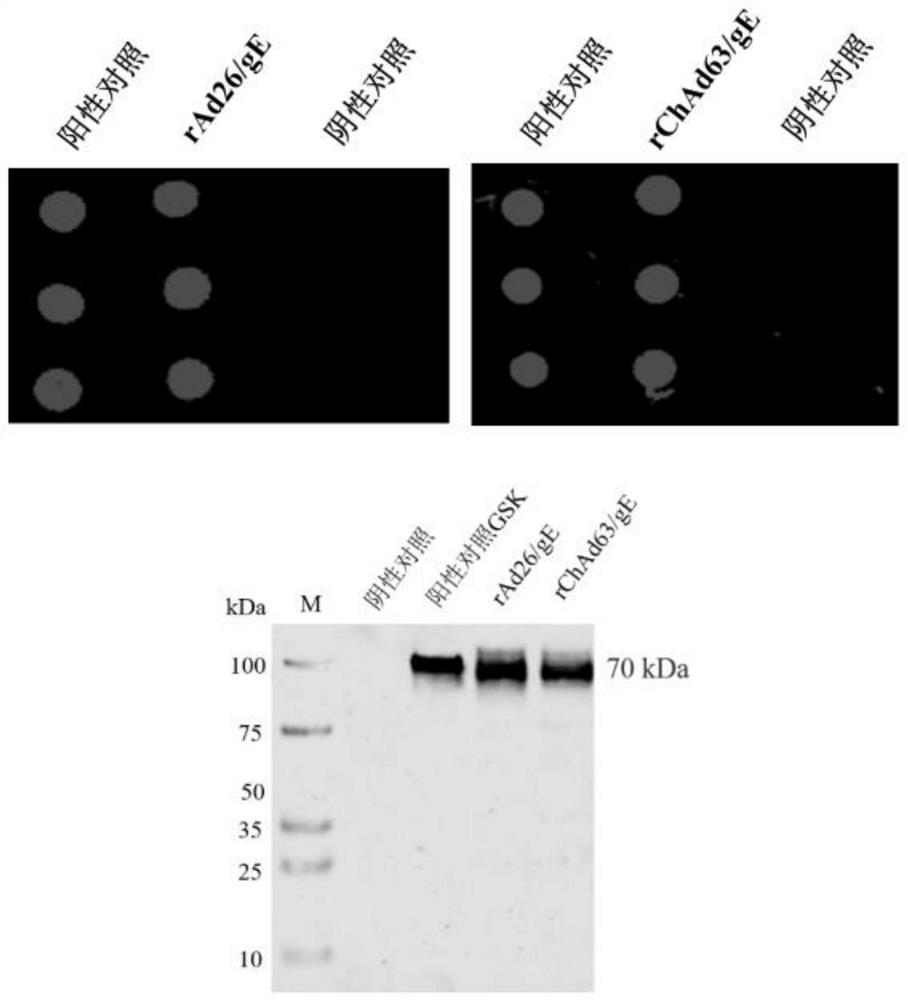
[0055] The recombinant adenoviral plasmid pAd26 / gE was digested with restriction endonucleases PacI and SpeI, and recovered by ethanol precipitation. The obtained linearized recombinant adenoviral DNA genome was transfected into 293 cells with Lipofectamine 2000, and the recombinant adenovirus rAd26 / gE expressing herpes zoster gE protein was rescued; the recombinant adenoviral plasmid pChAd63 was digested with restriction endonuclease PacI / gE, recovered by ethanol precipitation. The obtained linearized recombinant adenovirus DNA genomes were transfected into 293 cells with Lipofectamine 2000, and the recombinant adenovirus rChAd63 / gE expressing herpes zoster gE protein was rescued.
[0056] Take 2 μl each of the purified recombinant adenoviruses rAd26 / gE and rChAd63 / gE, inoculate them in 293 cells respectively, collect the cells after 80% of the cells have lesions, and use the gE protein monoclonal antib...
Embodiment 3
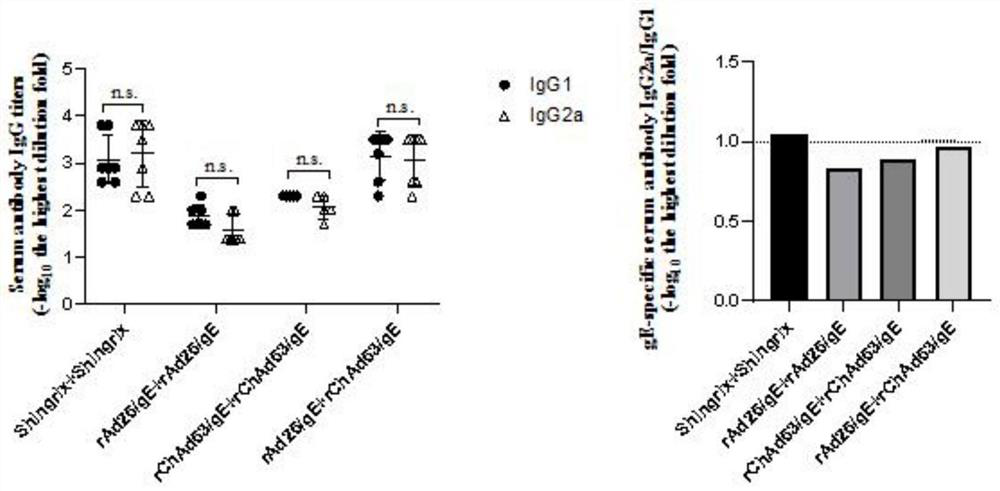
[0058] Shingles vaccine prime-boost
[0059] 1. Animal immunity
[0060] Female C57BL / 6 mice aged 6-8 weeks were divided into 5 groups, on the 0th day, the basic serum was collected, on the 1st day, the first intramuscular injection was immunized; on the 21st day after immunization, the second immunization was injected intramuscularly, twice intramuscular injection The dose was the same (Shingrix immunization dose was 5 μg / 50 μl / mouse, rAd26 / gE and rChAd63 / gE immunization dose was 10 8 PFU / 100 μl / mouse). On the 28th day, the rats were sacrificed to collect splenic lymphocytes and post-immunization serum.
[0061] The grouping and processing are as follows:
[0062] The first group (G1 group): negative control (PBS priming-PBS strengthening group);
[0063] The second group (G2 group): positive control (Shingrix priming-Shingrix boosting group);
[0064] The third group (G3 group): rAd26 / gE priming-rAd26 / gE boosting group;
[0065] The fourth group (G4 group): rChAd63 / gE ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com